PART 3 -----COLORIMETRIC VISUAL TESTING METHODS (What We See)
SOURCES OF ERROR IN THE ASSESSMENT PROCEDURE
Next we turn to the second component of our testing protocols and that is the ASSESSMENT…What do we see and how do we interpret the results of our testing procedure.
Visual colorimetric testing method requires the determination of either the color intensity (light pink vs. deep pink) or the color or hue (pink vs. blue) of the test solution. This means we need to understand sources of error associates with how we perceive color.
Human color perception is a complicated process and is a science unto itself. By definition color is what we see. The physical modification of light by an object as observed by the human eye and interpreted by the brain. I will not attempt to cover it in detail but will focus on the potential error sources in each of the 3 major components of human visual perception.
THE LIGHT SOURCE
The first component impacting human color perception is the LIGHT SOURCE Light is a form of energy and is part of a continuous energy spectrum going from Cosmic Rays to Electric Power. The portion of interest to us is the electromagnetic radiation between 380nm to 780nm. This is the portion of the spectrum that the human visual system is designed to responds to. The main issue with the light source is it needs to contain all of the radiation between 380-780nm. This is often referred to as a full spectrum continuous light source (Fig 7). Where as a light source that does not contain all of the radiation is referred to as a discontinuous source (Fig 8). In order to get accurate color rendition a full spectrum source is required as well as a high level of illumination.

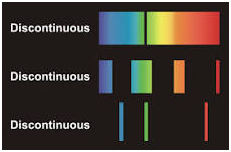
FIG 7 FIG 8
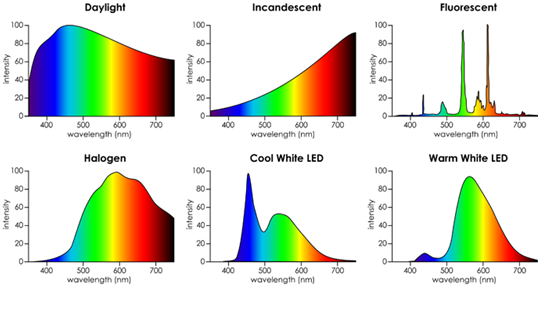
FIG 9
Figure 10 is an illustration of the difference that a light source can make on the appearance of the object.
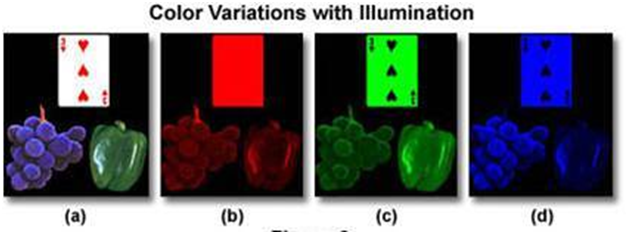
FIG 10
The following examples in Fig 11 and 12 illustrate how the difference in the lighting spectrum impacts what we see when looking at color standards for test kits.
 SOURCE A
SOURCE A
 SOURCE B
SOURCE B
FIG 11
SOURCE A SOURCE B
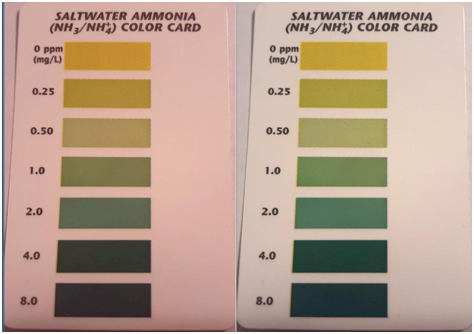
FIG 12
In both of these figures the same card is illuminated with two different light sources (A&B). A person with normal color vision would see that there is a visual difference between the two samples. This is a direct result of how the objects interact with the light source. This same issue plays out with titration colors as Fig 13 Illustrates

Illuminated Under Full Spectrum Source

Illuminated Under A Red-Yellow Source---Lacking Blue
FIG 13
THE OBJECTS
The second component impacting human color perception is the OBJECT we are viewing. Objects modify light based on their physical and chemical makeup. The interaction of an object with light can be characterized by its spectral reflectance (% Reflectance) & or transmission (% Transmission) curve. This curve tells us how a particular object interacts with our light source. If two objects have the same curve they are said to be a “color match”. This is what we are attempting to do when we compare our test solution to the color card or titrant color. We are looking for the closest color match to determine our results. As many will attest this can sometimes be a challenging step. The human visual system is capable of distinguishing very small color differences; however there is a lower limit to this ability. This lower limit is what sometimes makes it difficult to distinguish between different levels of test results. An example would be the card in figure 11. The difference between .01 and .03 could be noticeable but the difference between .01 and .02 or .03 and .04 may not be so easy.
There is another component of the objects that most people are not aware of, but can have a significant impact on what we see. When two objects (Test sample and color card) form what is called a metameric pair. This occurs when the two objects do not interact with light in the same way. The outcome of this would be that when we look at the samples under one light source they may look the same but when we view them under a second light source they will look different. Figures 14-17 illustrate this property.

FIG 14
FIG 15
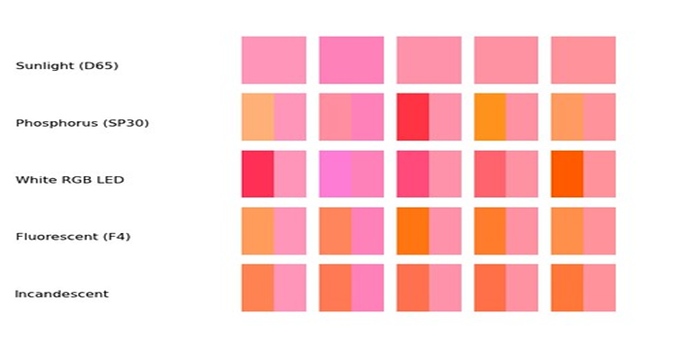
FIG 16
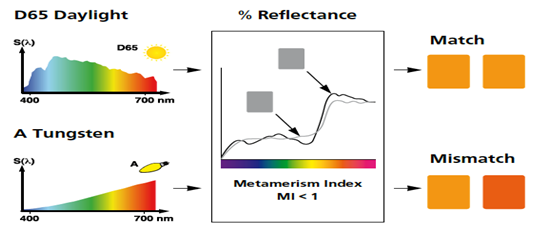
FIG 17
As you can see from these figures the colors of the two samples appear to be a match under one light source but they do not match under the other. Looking at figure 16 we can see the cause of this phenomenon. They do not have the same Spectral Reflectance curve!...They interact with light differently. This is condition generally occurs when the two samples have a different physical & or chemical makeup..Like our color card and test solutions. How does this impact aquarium water testing? Many of the test kits require the comparison of a “Standard” color card to a solution obtained from some type of reaction. The reaction yields a color based on the level of ion or element we are testing for. This necessarily means we are comparing a liquid solution to a card made from paint or ink. Paints and inks are colored with pigments and dyes blended in combination to produce the desired color. The chances are very high that the solution and the card made from pigments or dyes are not chemically the same. This means there is a high probability they will interact with light in different ways…Their spectral curves will be different. This means the possibility of Metamerism is high. This is why in some cases the test kit manufacture recommends a specific type of light source to make the evaluation. If the correct light source is not used the result could be that the observed color differences are not due to the test results but only to the fact the two samples are metameric. This could lead to an incorrect conclusion on the test results. Thus the importance of using the correct light source! For more details of Metamerism just GOOGLE “Metamerism in Color”, you will find lots of information.
THE HUMAN OBSERVER
The third component of the assessment procedure where errors can occur is with the human observer. The human visual system is quite remarkable and complex and is a science unto itself. I will cover two factors to consider where errors can occur.
First would be the fact that not everyone has “normal” color vision. There is a percentage of the population that has what is termed “color deficiencies”. It is the condition where receptors, called cones located in the back of the eye (See Fig 18) do not function properly. We have 3 types of receptors (cones), Red, Green & Blue. If these receptors are damaged or not functioning properly it leads to 3 general types of color vision deficiencies. Deuteranopia (green deficient), Protanopia (red deficient) and Tritanopia (blue deficient). About 8% of men and less than 1% of women have one or more of these conditions. Some estimates actually place these higher (20% of men) when considering that some have “mild” cases and are not completely “color” blind. Here is a good link to get more detail http://www.colourblindawareness.org/colour-blindness/ . Fig 19 shows what the visual impact of this condition.
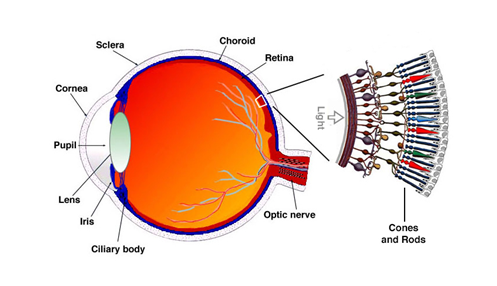
FIG 18
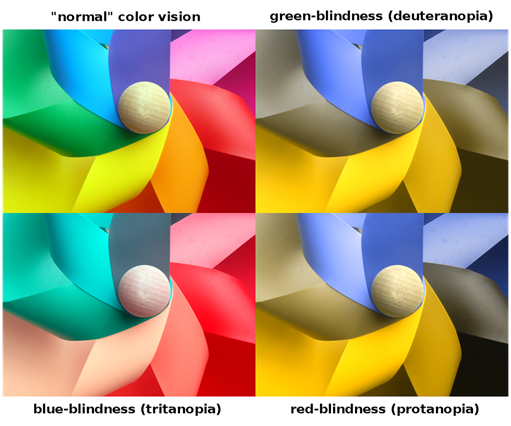
FIG 19
You can see (if you have normal color vision) from Fig 19 that the impact on making color evaluation would be hindered. When coupled with the problem of Metamerism it can become very difficult to correctly distinguish small color differences or determine the end point of a titration. The second factor that can contribute to errors is age. As we age the crystalline lens (See fig 17) begins to yellow. This has the effect of filtering out blue and some portion of the green spectra. This means we see less blue in the samples we are observing. This makes the distinguishing of subtle color differences more difficult especially if the sample has a small blue component. Again this problem is compounded with the problem of Metamerism.
Just as a side note. UV activated lenses used in some eye glasses can also cause visual assessment errors when evaluation test results. If the light source has enough UV present it will activate the lenses and they will filter out some of the blue end of the spectrum.
CONCLUSIONS ON VISUAL TESTING METHODS
The potential sources of error in Visual Testing are numerous, both with regard to Evaluation (Test Procedures) & Assessment. The good news is by following some “Best Practices” one can get good Precision and Accuracy. The following is a list of some of the “Best Practices” that can help achieve this.
TEST KIT: MAGNESIUM
3 individual tests were conducted on the same water sample. The test was repeated on 3 consecutive days resulting in 9 measurements of the same sample. Two different procedures were used. The Standard procedure using the equipment provided in the test kit. The level of care in doing the procedure was normal.
In this example by changing 5 components of the test the Relative Error and Relative Standard Deviation were reduced thus yielding more reliable results. The 5 components that were changed were:
The advantage of having a more reliable result is you can have more confidence in a single measurement. As you can see from table 7 although the relative errors are not large the range (High to Low) is fairly big 225ppm. This would mean less confidence in any single measurement and the potential necessity to take several measurements and take the average to reduce the error. In addition if you have a known standard to run your test against and you have low % relative standard deviation you can apply a correction factor to “true up” your results. In the above example (Table 8) the average of all 9 samples is 1320ppm. If we take the ICP results as the actual value 1355ppm and subtract the average 1320ppm we get a correction factor of 35ppm.Adding this to our highest and lowest measurement in table 7 yield results of 1335ppm to 1365ppm from the low to the high measurement….10-20ppm off the actual value…A pretty tight range…This would not work for the data in table 7…Average 1287ppm a difference of 68ppm from the actual value…Adding this to the low and high values that would give us values of 1308ppm to 1533ppm or 47ppm to 178ppm off the actual value…much bigger error, enough to cause a high level of uncertainty in the measurement.
This is only one example of the value of enhancing the Accuracy and Precision of our tests. This can be applied to all of our aquarium testing and increase our confidence in the results and there by the confidence in managing our tanks.
This concludes PART 3 of our journey into aquarium water testing from a Quality System Mind Set…Measure-----> Evaluate-----> Improve—-->New Method.
In PART 4 will cover Colorimetric Instrumental (Digital) Testing Methods and review some aspects of ICP Testing.
SOURCES OF ERROR IN THE ASSESSMENT PROCEDURE
Next we turn to the second component of our testing protocols and that is the ASSESSMENT…What do we see and how do we interpret the results of our testing procedure.
Visual colorimetric testing method requires the determination of either the color intensity (light pink vs. deep pink) or the color or hue (pink vs. blue) of the test solution. This means we need to understand sources of error associates with how we perceive color.
Human color perception is a complicated process and is a science unto itself. By definition color is what we see. The physical modification of light by an object as observed by the human eye and interpreted by the brain. I will not attempt to cover it in detail but will focus on the potential error sources in each of the 3 major components of human visual perception.
1) The LIGHT SOURCE
2) THE OBJECT
3) THE OBSERVER (Human visual system)
THE LIGHT SOURCE
The first component impacting human color perception is the LIGHT SOURCE Light is a form of energy and is part of a continuous energy spectrum going from Cosmic Rays to Electric Power. The portion of interest to us is the electromagnetic radiation between 380nm to 780nm. This is the portion of the spectrum that the human visual system is designed to responds to. The main issue with the light source is it needs to contain all of the radiation between 380-780nm. This is often referred to as a full spectrum continuous light source (Fig 7). Where as a light source that does not contain all of the radiation is referred to as a discontinuous source (Fig 8). In order to get accurate color rendition a full spectrum source is required as well as a high level of illumination.
FIG 7 FIG 8
Fig 9 is a chart of 6 light sources and their spectral energy distributions. There are many more light sources but this demonstrates that each source has a different spectral make up and thus can render the color of an object (color card or solution) differently. It is especially notable that the Fluorescent light source is missing (or they are very low) portions of the visable spectrum. This will render the color of the object much differently than the other sources. This is why full spectrum florescent lamps should be used for color evaluation.FIG 9
Figure 10 is an illustration of the difference that a light source can make on the appearance of the object.
FIG 10
(a) Full spectrum light source containing wavelengths of light in the visible spectrum
(b) Light source containing only red portion of the visible spectrum
(c) Light source containing only green portion of the visible spectrum
(d) Light source containing only blue portion of the visible spectrum
As you can see the objects appear significantly different under the different light sources.The implication for visual evaluation is the light source plays a key role in what you observe.The following examples in Fig 11 and 12 illustrate how the difference in the lighting spectrum impacts what we see when looking at color standards for test kits.
FIG 11
SOURCE A SOURCE B
FIG 12
In both of these figures the same card is illuminated with two different light sources (A&B). A person with normal color vision would see that there is a visual difference between the two samples. This is a direct result of how the objects interact with the light source. This same issue plays out with titration colors as Fig 13 Illustrates
Illuminated Under Full Spectrum Source
Illuminated Under A Red-Yellow Source---Lacking Blue
FIG 13
The second component impacting human color perception is the OBJECT we are viewing. Objects modify light based on their physical and chemical makeup. The interaction of an object with light can be characterized by its spectral reflectance (% Reflectance) & or transmission (% Transmission) curve. This curve tells us how a particular object interacts with our light source. If two objects have the same curve they are said to be a “color match”. This is what we are attempting to do when we compare our test solution to the color card or titrant color. We are looking for the closest color match to determine our results. As many will attest this can sometimes be a challenging step. The human visual system is capable of distinguishing very small color differences; however there is a lower limit to this ability. This lower limit is what sometimes makes it difficult to distinguish between different levels of test results. An example would be the card in figure 11. The difference between .01 and .03 could be noticeable but the difference between .01 and .02 or .03 and .04 may not be so easy.
There is another component of the objects that most people are not aware of, but can have a significant impact on what we see. When two objects (Test sample and color card) form what is called a metameric pair. This occurs when the two objects do not interact with light in the same way. The outcome of this would be that when we look at the samples under one light source they may look the same but when we view them under a second light source they will look different. Figures 14-17 illustrate this property.
FIG 14
FIG 15
FIG 16
FIG 17
As you can see from these figures the colors of the two samples appear to be a match under one light source but they do not match under the other. Looking at figure 16 we can see the cause of this phenomenon. They do not have the same Spectral Reflectance curve!...They interact with light differently. This is condition generally occurs when the two samples have a different physical & or chemical makeup..Like our color card and test solutions. How does this impact aquarium water testing? Many of the test kits require the comparison of a “Standard” color card to a solution obtained from some type of reaction. The reaction yields a color based on the level of ion or element we are testing for. This necessarily means we are comparing a liquid solution to a card made from paint or ink. Paints and inks are colored with pigments and dyes blended in combination to produce the desired color. The chances are very high that the solution and the card made from pigments or dyes are not chemically the same. This means there is a high probability they will interact with light in different ways…Their spectral curves will be different. This means the possibility of Metamerism is high. This is why in some cases the test kit manufacture recommends a specific type of light source to make the evaluation. If the correct light source is not used the result could be that the observed color differences are not due to the test results but only to the fact the two samples are metameric. This could lead to an incorrect conclusion on the test results. Thus the importance of using the correct light source! For more details of Metamerism just GOOGLE “Metamerism in Color”, you will find lots of information.
THE HUMAN OBSERVER
The third component of the assessment procedure where errors can occur is with the human observer. The human visual system is quite remarkable and complex and is a science unto itself. I will cover two factors to consider where errors can occur.
First would be the fact that not everyone has “normal” color vision. There is a percentage of the population that has what is termed “color deficiencies”. It is the condition where receptors, called cones located in the back of the eye (See Fig 18) do not function properly. We have 3 types of receptors (cones), Red, Green & Blue. If these receptors are damaged or not functioning properly it leads to 3 general types of color vision deficiencies. Deuteranopia (green deficient), Protanopia (red deficient) and Tritanopia (blue deficient). About 8% of men and less than 1% of women have one or more of these conditions. Some estimates actually place these higher (20% of men) when considering that some have “mild” cases and are not completely “color” blind. Here is a good link to get more detail http://www.colourblindawareness.org/colour-blindness/ . Fig 19 shows what the visual impact of this condition.
FIG 18
FIG 19
You can see (if you have normal color vision) from Fig 19 that the impact on making color evaluation would be hindered. When coupled with the problem of Metamerism it can become very difficult to correctly distinguish small color differences or determine the end point of a titration. The second factor that can contribute to errors is age. As we age the crystalline lens (See fig 17) begins to yellow. This has the effect of filtering out blue and some portion of the green spectra. This means we see less blue in the samples we are observing. This makes the distinguishing of subtle color differences more difficult especially if the sample has a small blue component. Again this problem is compounded with the problem of Metamerism.
Just as a side note. UV activated lenses used in some eye glasses can also cause visual assessment errors when evaluation test results. If the light source has enough UV present it will activate the lenses and they will filter out some of the blue end of the spectrum.
CONCLUSIONS ON VISUAL TESTING METHODS
The potential sources of error in Visual Testing are numerous, both with regard to Evaluation (Test Procedures) & Assessment. The good news is by following some “Best Practices” one can get good Precision and Accuracy. The following is a list of some of the “Best Practices” that can help achieve this.
1) Establish Good Laboratory Practices. (EVALUATION)
a. Clean Equipment—Use dedicated test vials—Don’t use the same vial for different test…We will cover this in more detail later in Part 4
b. Accurate-Repeatable Measuring Devices…Droppers, Measures, Pipettes
c. Keep all equipment clean and rinsed (Remember 3 times rinse with RODI water)
d. It is also good practice to rinse out vials/syringes/pipettes with the water to be tested before conduction the test.
e. Read The Test Kit Instructions
f. Re-Read The Test Kit Instructions (look for “Tips in Using the Kit)
g. Follow The Test Kit Instructions
h. Assure the Test Kit is not expired!
i. Be consistent from test to test on each step of the instructions
j. Use a timer to time steps that call for it.
k. Run The Test 3-5 Times on The Same Sample to Check Repeatability…Know the limits of variability of the Test % Relative Error & Standard Deviation
l. If at all possible run the test on a “Known” set of samples to gage the Accuracy of the test.
m. Test at same time of day
n. Test in a controlled environment….Temperature, Humidity Lighting
o. Check to see that a new set of reagents gives the similar results as the previous batch.
2) Looking at the test results and making a determination (ASSESSMENT)
a. Select a light source that is full spectrum & or use the light source recommended by the test kit manufacture.
b. Determine if you color vision is normal. There are several websites where color vision can be checked… Below are some;
c. Make sure the background that you are looking at your samples against is a neutral as possible. Brightly colored tables or benches can impact color judgment.
d. The manufacture of the test kit will sometimes give “Tips” to get better results. This could include the best way to observe the sample to get more accurate results.
Spending the time to reduce or eliminate the many sources of error will produce more Accuracy and Precision in testing your aquarium’s water. This will give more assurance about any adjustments being made as a result of your test. I have provided the following example to demonstrate the value of this thinking.TEST KIT: MAGNESIUM
3 individual tests were conducted on the same water sample. The test was repeated on 3 consecutive days resulting in 9 measurements of the same sample. Two different procedures were used. The Standard procedure using the equipment provided in the test kit. The level of care in doing the procedure was normal.
STANDARD PROCEDURE
TEST # | TEST 1 | TEST 2 | TEST 3 | % RELATIVE ERROR | % RELATIVE STDEV |
DAY 1 | 1300 | 1285 | 1465 | .4% | 7.4% |
DAY 2 | 1240 | 1255 | 1300 | 6.6% | 2.5% |
DAY 3 | 1270 | 1225 | 1240 | 8.1% | 1.8% |
ICP RESULTS | 1355 | 1355 | 1355 | |
TABLE 8
With all 9 measurements the Relative Error is 5%-- Relative Standard Deviation is 7%
ENHANCED PROCEDURE
With all 9 measurements the Relative Error is 5%-- Relative Standard Deviation is 7%
ENHANCED PROCEDURE
TEST # | TEST 1 | TEST 2 | TEST 3 | % RELATIVE ERROR | % RELATIVE STDEV |
DAY 1 | 1330 | 1315 | 1315 | 2.6% | .7% |
DAY 2 | 1315 | 1300 | 1330 | 3% | 1.1% |
DAY 3 | 1330 | 1315 | 1330 | 2.2% | .65% |
ICP RESULTS | 1355 | 1355 | 1355 | |
With all 9 measurements the Relative Error is 2.6%-- Relative Standard Deviation is .8%
In this example by changing 5 components of the test the Relative Error and Relative Standard Deviation were reduced thus yielding more reliable results. The 5 components that were changed were:
1) Using a more accurate Pipette (not syringe) to do the titration
2) Using a pipette to dispense liquid reagent and not using dropper.
3) Leveling out the dry component measurement
4) Replacing the plastic vile with glass vile and cleaning it with .1M HCL between uses.
5) Followed all of the good Laboratory Practices described above
The advantage of having a more reliable result is you can have more confidence in a single measurement. As you can see from table 7 although the relative errors are not large the range (High to Low) is fairly big 225ppm. This would mean less confidence in any single measurement and the potential necessity to take several measurements and take the average to reduce the error. In addition if you have a known standard to run your test against and you have low % relative standard deviation you can apply a correction factor to “true up” your results. In the above example (Table 8) the average of all 9 samples is 1320ppm. If we take the ICP results as the actual value 1355ppm and subtract the average 1320ppm we get a correction factor of 35ppm.Adding this to our highest and lowest measurement in table 7 yield results of 1335ppm to 1365ppm from the low to the high measurement….10-20ppm off the actual value…A pretty tight range…This would not work for the data in table 7…Average 1287ppm a difference of 68ppm from the actual value…Adding this to the low and high values that would give us values of 1308ppm to 1533ppm or 47ppm to 178ppm off the actual value…much bigger error, enough to cause a high level of uncertainty in the measurement.
This is only one example of the value of enhancing the Accuracy and Precision of our tests. This can be applied to all of our aquarium testing and increase our confidence in the results and there by the confidence in managing our tanks.
This concludes PART 3 of our journey into aquarium water testing from a Quality System Mind Set…Measure-----> Evaluate-----> Improve—-->New Method.