- Joined
- Sep 21, 2018
- Messages
- 6,654
- Reaction score
- 7,142
Aragonite adsorption of phosphate is probably of interest to anyone who maintains a saltwater aquarium. There is a notion in the hobby that aragonite sand and rocks can bind huge amounts of phosphate that can later be released to the aquarium and raise the amount of phosphate in the water to an undesirable level. I recently had a need to know precisely how much and how fast phosphate binds to aragonite sand. I searched the internet for the information but ended up going to the lab anyway
(https://www.reef2reef.com/threads/aragonite-sand-and-phosphate-adsorption.944916/).
In this post, I summarize some new observations and their implications for the marine aquarium hobby.
How Much Phosphate Binds To Aragonite?
Mineral type (aragonite or calcite) and surface area play important roles in determining phosphate adsorption capacity. Temperature, salinity and temperature also have significant effects. Aragonite may have more phosphate binding capacity than calcite (Millero). As for surface area, fine sand will bind more phosphate than coarse sand and chunks of aragonite. Since there are so many variables involved in predicting aragonite behavior in the lab, I decided to observe phosphate adsorption under experimental conditions that I plan to use in future experiments.
My observations of phosphate adsorption were made with CaribsSea aragonite that I will use in future experiments. The product “aragamix sugar size oolite” is a very fine sand. Based on sand grain size, the surface area should be at least 20-40 cm^2 per gram. So, how much phosphate is adsorbed by aragonite sugar sand? How much phosphate can desorb or leach from the sand afterwards?
Adsorption And Desorption Of Phosphate
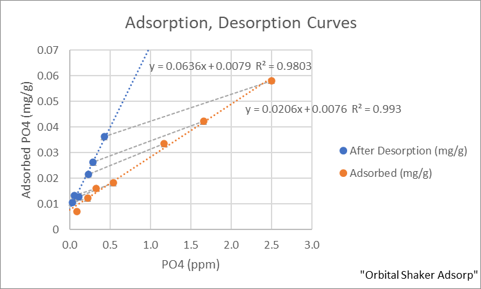
How much phosphate is adsorbed depends on the concentration of phosphate in the water. Mixing sand with a phosphate in Instant Ocean results in a decrease in the phosphate concentration. The difference between the initial and final concentration represents the amount of phosphate adsorbed by the sand. For example, if a 25 mL solution of 2 ppm phosphate after mixing with 0.4 g of sand becomes 1.4 ppm, the amount of phosphate removed by the sand from solution is 0.6 ppm, or 0.6 mg/L * 0.025 L = 0.015 mg phosphate removed. Since 0.4 g of sand was present, 0.038 mg of phosphate was adsorbed per g of aragonite sand at 1.4 ppm phosphate. The orange line in the plot below is the aragonite sugar sand adsorption curve for a range of phosphate concentrations. The desorption curve (the blue line) represents how much phosphate remains on the sand when it is mixed with phosphate free Instant Ocean for twenty four hours. The dashed tie lines between the adsorption and desorption lines connect the data derived from the same sample of sand. The blue and orange lines not overlapping tells us that the experimental data looks like the phosphate is not equilibrium between sand and water, maybe being irreversibly bound. The following example further illustrates the problem.
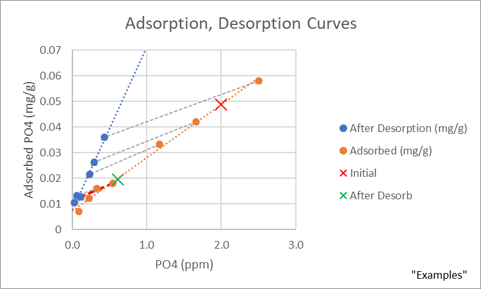
On the adsorption line below, I show with “X’s” how phosphate in equilibrium responds when that equilibrium is disturbed. The red “X” marks a position on the adsorption curve for a sand sample in equilibrium with 2 ppm phosphate. If that sand was moved from the 2 ppm phosphate solution to phosphate free seawater, the sand should release phosphate to the seawater until the new equilibrium was established. This point is marked with a green “X” at 0.61 ppm phosphate, which is below the observed desorption line. This is actually good news for aquarists. Sand releases less adsorbed phosphate than we feared. Is this really true though? Extraordinary claims need extraordinary evidence.
To confirm the observation that aragonite sand can irreversibly bind phosphate, a sample of sand was exposed to 2 ppm phosphate in Instant Ocean for twenty four hours and then the sand was stirred multiple times with 0 ppm phosphate Instant Ocean until no more phosphate desorbed. This cycle was repeated three more times. This should show whether phosphate accumulates irreversibly. Another four samples of sand were exposed to the same 2 ppm solution of phosphate for twenty four hours, but instead of desorbing the phosphate, the medium was replaced with fresh 2 ppm phosphate every twenty four hours. One sand sample was sacrificed each twenty four hours to completely desorb the phosphate. This experiment will show whether irreversible accumulation occurs over time at a constant concentration of phosphate. In the following plots of the data, the first experiment is labeled “Ad-De” and the second “Ad”.
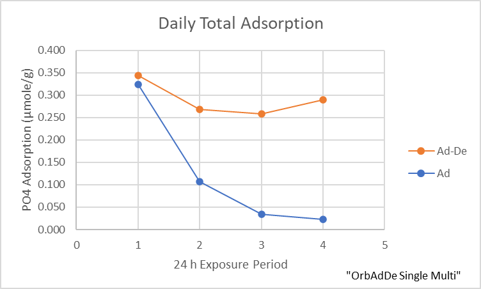
In the “Ad-De” experiment, the adsorption of phosphate decreases about 0.1 micro moles per gram of sand, or ~0.01 mg/g by the second adsorption-desorption cycle and then the decline levels off. The adsorption capacity is renewable and stable at about .25 micro moles/g or about 0.025 mg/g at about 1.7 ppm. This is less than indicated by the adsorption line, possibly because the line was constructed with data that included both reversible and irreversible bound phosphate. The adsorption in the “Ad” experiment declined 0.35 micro moles/g or ~0.035 mg/g likely because the sand had reached an equilibrium with the 2 ppm phosphate solution and stopped adsorbing phosphate. Adding the daily adsorption amounts in the “Ad” experiment, a grand total of 0.49 micro moles/g or ~.05 mg/g phosphate was adsorbed at 2 ppm PO4. This value is in agreement with the adsorption line, possibly because the result contains both reversible and irreversibly bound phosphate.
Subtracting the amount of phosphate that desorbs from the total amount of adsorbed phosphate (see next plot) reveals that irreversibly bound phosphate accumulates on the sand that underwent repeated adsorption-desorption cycles (~0.08 mg/g) but probably very little if any beyond the initial level by just being at equilibrium with 2 ppm phosphate 4 days.
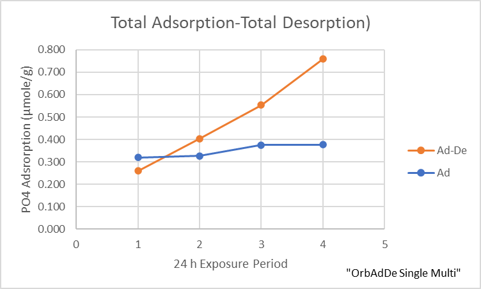
This data suggests to me that irreversibly bound phosphate is generated only during the adsorption process and no more is generated by continued exposure of the sand to phosphate. Further, after removal of the reversibly bound phosphate through exhaustive desorption, the surface capacity for both types of phosphate binding seems unaffected by previous adsorption events.
Marine Aquarium Hobby Implications
Life goes on even in light of this new data. Aragonite adsorbs phosphate (not as much as we thought) and lets it go (not as much as we thought). Period. What possibly changes is our understanding of some hobby processes.
Rip Cleaning. This is an aggressive aquarium cleaning method that involves the complete removal of an aquarium substrate and thorough rinsing in tap water to remove accumulated organic material and living organisms. When the tap water contains phosphate, does such a cleaning process add phosphate to the substrate that can leach into the aquarium water? As a first approximation the answer is yes, but we should also ask how much and what might we do to reap the benefits of rip cleaning without the downside of phosphate adsorption.
From the observations above, we know only so much phosphate can bind to an aragonite surface for a given concentration of phosphate in the tap water, and the amount is not as much as we thought, maybe only 1-10%. And while the amount that desorbs can raise the phosphate level of aquarium water, if the substrate is thoroughly stirred in phosphate free water several times (exhaustive desorption), it is possible to remove all of the reversibly bound phosphate. To know if phosphate adsorption after rip cleaning is even an issue, stirring a small amount of rip cleaned sand in phosphate free saltwater for 15-30 minutes and then testing for phosphate answers the question. If the ratio of sand to water in the test is the same as that in the aquarium, the observed phosphate concentration will be the same to what it will be in the reassembled aquarium. If the ratio was different, some math would be needed to predict what will happen in the aquarium.
Leaching Phosphate. Very likely you have read about phosphate leaching from aragonite takes a very long time and how much there must be. The observation about the desorption rate is real, the conclusion drawn about the large amount of phosphate might be a misinterpretation. Reversibly bound phosphate rapidly desorbs from aragonite, requiring less than thirty minutes to reach equilibrium in well stirred water. Achieving this level of mixing is difficult in a bucket full of rocks equipped with only a powerhead. The less than thirty minute desorption rate is now stretched to hours because of stagnant water around the rocks. Also, water in the bucket is typically not changed that often in the leaching process, causing the desorption to stop and the phosphate to seemingly take forever to leach from the rocks. Another possible mechanism invoked to explain a slow leaching process is the slow diffusion of phosphate from porous rocks. While this is certainly possible, there is no need to invoke this line of reasoning in light of the inefficiency of the standard desorption process.
Relative Surface Area Determination. I wonder if we might use the amount of phosphate adsorbed to aragonite as a way to obtain a relative surface area determination. By measuring the adsorption rate for an aragonite reference standard of known surface area per gram, we could then compare it to the phosphate adsorption rate for a material with an unknown surface area to estimate its surface area per gram. Validating the method would be a pain.
Hope this post wasn’t too confusing. Comments and suggestions welcome.
Dan
Millero Reference
Adsorption and Desorption of Phosphate on Calcite and Aragonite in Seawater
Aquatic Geochemistry 7: 33–56, 2001, Kluwer Academic Publishers
FRANK MILLERO1, FEN HUANG1, XIAORONG ZHU1, XUEWU LIU1 and JIA-ZHONG ZHANG2
(https://www.reef2reef.com/threads/aragonite-sand-and-phosphate-adsorption.944916/).
In this post, I summarize some new observations and their implications for the marine aquarium hobby.
How Much Phosphate Binds To Aragonite?
Mineral type (aragonite or calcite) and surface area play important roles in determining phosphate adsorption capacity. Temperature, salinity and temperature also have significant effects. Aragonite may have more phosphate binding capacity than calcite (Millero). As for surface area, fine sand will bind more phosphate than coarse sand and chunks of aragonite. Since there are so many variables involved in predicting aragonite behavior in the lab, I decided to observe phosphate adsorption under experimental conditions that I plan to use in future experiments.
My observations of phosphate adsorption were made with CaribsSea aragonite that I will use in future experiments. The product “aragamix sugar size oolite” is a very fine sand. Based on sand grain size, the surface area should be at least 20-40 cm^2 per gram. So, how much phosphate is adsorbed by aragonite sugar sand? How much phosphate can desorb or leach from the sand afterwards?
Adsorption And Desorption Of Phosphate
How much phosphate is adsorbed depends on the concentration of phosphate in the water. Mixing sand with a phosphate in Instant Ocean results in a decrease in the phosphate concentration. The difference between the initial and final concentration represents the amount of phosphate adsorbed by the sand. For example, if a 25 mL solution of 2 ppm phosphate after mixing with 0.4 g of sand becomes 1.4 ppm, the amount of phosphate removed by the sand from solution is 0.6 ppm, or 0.6 mg/L * 0.025 L = 0.015 mg phosphate removed. Since 0.4 g of sand was present, 0.038 mg of phosphate was adsorbed per g of aragonite sand at 1.4 ppm phosphate. The orange line in the plot below is the aragonite sugar sand adsorption curve for a range of phosphate concentrations. The desorption curve (the blue line) represents how much phosphate remains on the sand when it is mixed with phosphate free Instant Ocean for twenty four hours. The dashed tie lines between the adsorption and desorption lines connect the data derived from the same sample of sand. The blue and orange lines not overlapping tells us that the experimental data looks like the phosphate is not equilibrium between sand and water, maybe being irreversibly bound. The following example further illustrates the problem.
On the adsorption line below, I show with “X’s” how phosphate in equilibrium responds when that equilibrium is disturbed. The red “X” marks a position on the adsorption curve for a sand sample in equilibrium with 2 ppm phosphate. If that sand was moved from the 2 ppm phosphate solution to phosphate free seawater, the sand should release phosphate to the seawater until the new equilibrium was established. This point is marked with a green “X” at 0.61 ppm phosphate, which is below the observed desorption line. This is actually good news for aquarists. Sand releases less adsorbed phosphate than we feared. Is this really true though? Extraordinary claims need extraordinary evidence.
To confirm the observation that aragonite sand can irreversibly bind phosphate, a sample of sand was exposed to 2 ppm phosphate in Instant Ocean for twenty four hours and then the sand was stirred multiple times with 0 ppm phosphate Instant Ocean until no more phosphate desorbed. This cycle was repeated three more times. This should show whether phosphate accumulates irreversibly. Another four samples of sand were exposed to the same 2 ppm solution of phosphate for twenty four hours, but instead of desorbing the phosphate, the medium was replaced with fresh 2 ppm phosphate every twenty four hours. One sand sample was sacrificed each twenty four hours to completely desorb the phosphate. This experiment will show whether irreversible accumulation occurs over time at a constant concentration of phosphate. In the following plots of the data, the first experiment is labeled “Ad-De” and the second “Ad”.
In the “Ad-De” experiment, the adsorption of phosphate decreases about 0.1 micro moles per gram of sand, or ~0.01 mg/g by the second adsorption-desorption cycle and then the decline levels off. The adsorption capacity is renewable and stable at about .25 micro moles/g or about 0.025 mg/g at about 1.7 ppm. This is less than indicated by the adsorption line, possibly because the line was constructed with data that included both reversible and irreversible bound phosphate. The adsorption in the “Ad” experiment declined 0.35 micro moles/g or ~0.035 mg/g likely because the sand had reached an equilibrium with the 2 ppm phosphate solution and stopped adsorbing phosphate. Adding the daily adsorption amounts in the “Ad” experiment, a grand total of 0.49 micro moles/g or ~.05 mg/g phosphate was adsorbed at 2 ppm PO4. This value is in agreement with the adsorption line, possibly because the result contains both reversible and irreversibly bound phosphate.
Subtracting the amount of phosphate that desorbs from the total amount of adsorbed phosphate (see next plot) reveals that irreversibly bound phosphate accumulates on the sand that underwent repeated adsorption-desorption cycles (~0.08 mg/g) but probably very little if any beyond the initial level by just being at equilibrium with 2 ppm phosphate 4 days.
This data suggests to me that irreversibly bound phosphate is generated only during the adsorption process and no more is generated by continued exposure of the sand to phosphate. Further, after removal of the reversibly bound phosphate through exhaustive desorption, the surface capacity for both types of phosphate binding seems unaffected by previous adsorption events.
Marine Aquarium Hobby Implications
Life goes on even in light of this new data. Aragonite adsorbs phosphate (not as much as we thought) and lets it go (not as much as we thought). Period. What possibly changes is our understanding of some hobby processes.
Rip Cleaning. This is an aggressive aquarium cleaning method that involves the complete removal of an aquarium substrate and thorough rinsing in tap water to remove accumulated organic material and living organisms. When the tap water contains phosphate, does such a cleaning process add phosphate to the substrate that can leach into the aquarium water? As a first approximation the answer is yes, but we should also ask how much and what might we do to reap the benefits of rip cleaning without the downside of phosphate adsorption.
From the observations above, we know only so much phosphate can bind to an aragonite surface for a given concentration of phosphate in the tap water, and the amount is not as much as we thought, maybe only 1-10%. And while the amount that desorbs can raise the phosphate level of aquarium water, if the substrate is thoroughly stirred in phosphate free water several times (exhaustive desorption), it is possible to remove all of the reversibly bound phosphate. To know if phosphate adsorption after rip cleaning is even an issue, stirring a small amount of rip cleaned sand in phosphate free saltwater for 15-30 minutes and then testing for phosphate answers the question. If the ratio of sand to water in the test is the same as that in the aquarium, the observed phosphate concentration will be the same to what it will be in the reassembled aquarium. If the ratio was different, some math would be needed to predict what will happen in the aquarium.
Leaching Phosphate. Very likely you have read about phosphate leaching from aragonite takes a very long time and how much there must be. The observation about the desorption rate is real, the conclusion drawn about the large amount of phosphate might be a misinterpretation. Reversibly bound phosphate rapidly desorbs from aragonite, requiring less than thirty minutes to reach equilibrium in well stirred water. Achieving this level of mixing is difficult in a bucket full of rocks equipped with only a powerhead. The less than thirty minute desorption rate is now stretched to hours because of stagnant water around the rocks. Also, water in the bucket is typically not changed that often in the leaching process, causing the desorption to stop and the phosphate to seemingly take forever to leach from the rocks. Another possible mechanism invoked to explain a slow leaching process is the slow diffusion of phosphate from porous rocks. While this is certainly possible, there is no need to invoke this line of reasoning in light of the inefficiency of the standard desorption process.
Relative Surface Area Determination. I wonder if we might use the amount of phosphate adsorbed to aragonite as a way to obtain a relative surface area determination. By measuring the adsorption rate for an aragonite reference standard of known surface area per gram, we could then compare it to the phosphate adsorption rate for a material with an unknown surface area to estimate its surface area per gram. Validating the method would be a pain.
Hope this post wasn’t too confusing. Comments and suggestions welcome.
Dan
Millero Reference
Adsorption and Desorption of Phosphate on Calcite and Aragonite in Seawater
Aquatic Geochemistry 7: 33–56, 2001, Kluwer Academic Publishers
FRANK MILLERO1, FEN HUANG1, XIAORONG ZHU1, XUEWU LIU1 and JIA-ZHONG ZHANG2















