- Joined
- Sep 21, 2018
- Messages
- 6,675
- Reaction score
- 7,169
In the near future, I will looking at how phosphate adsorbed to aragonite sand affects nuisance algae growth. I wondered about the phosphate molecules hopping on and off the sand grains. How fast does does sand bind PO4? How much phosphate does a gram of aragonite sugar sand adsorb? Does the bound PO4 come off? How fast does it come off? Here is a description of my experiments and results.
Methods. About 3 grams (measured) of washed CaribSea aragonite sand and 40 mL Instant Ocean containing phosphate were placed in a plastic 50 mL screw cap centrifuge tube and placed horizontally on an orbital shaker to gently rock the solution over the shallow bed of sand for 24 hours in the dark. The final phosphate concentration of the Instant Ocean solution was determined with the Hanna low range phosphate Checker. The difference between the initial and final concentrations corresponded to the amount of phosphate adsorbed by the sample of sand.
Desorption experiments were carried in the centrifuge tubes containing sand used in the absorption study and 40 mL 0 ppm phosphate Instant Ocean. The capped centrifuge tubes were placed horizontally on an orbital shaker for several days in the dark.
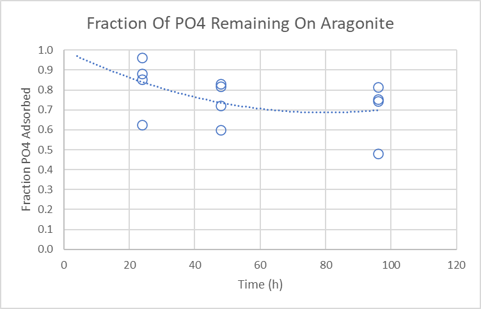
Results. Phosphate binding occurred within 6 hours but adsorption experiments were equilibrated for 24 hours to ensure the results were closer to equilibrium. The first plot below shows the amount of phosphate adsorbed at different equilibrium phosphate concentrations. The second plot shows that only a fraction of the adsorbed phosphate leaves the sand surface quickly. This is consistent with observations that removing surface bound phosphate from aragonite rocks requires a long wait.
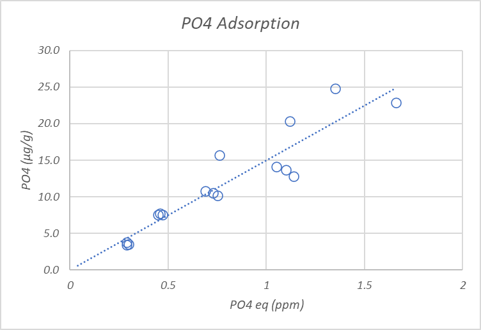
How do the adsorption results compare to the scientific literature (Millero, 2001) and the JDA study (Post #70 for results)? The chart below summarizes what we might guesstimate about how much phosphate is bound to 20 pounds of aragonite sand equilibrated at 0.5 ppm phosphate. Because adsorption is a surface phenomenon, the amount of available surface area per unit mass needs to be known to accurately predict the bound phosphate. We do not have this number for each material in the table but we can make some educated guesses and discuss the estimates.
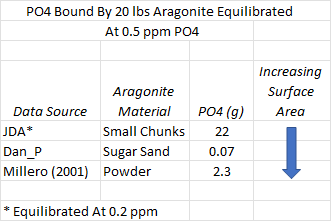
Millero synthesized aragonite and likely obtained a very fine precipitate. Similar synthesizes generated material with particles in the range of 50-100 microns. Materials with such a particle size in nature are classified as silt and estimated to have a surface area per gram of around 400 cm^2. The sand that I studied has particle sizes smaller than 1 mm, making it a fine to coarse sand with an estimated surface area per gram of 23-45 cm^2. With these crude estimates, the 10x range in surface area can explain much of the difference in adsorbed phosphate between my results and Millero’s. Can a surface area difference explain why large chunks of aragonite in JDA’s experiment adsorb so much more phosphate than Millero’s silty aragonite?
The simple answer is that the phosphate binding rate cannot exceed the limit observed by Millero, but lets think about this further. If the phosphate binding rate is 10X Millero’s, the surface area would have to be 10x higher or 4000 cm^2 per gram! Surface area could be larger than the visible surface area for a chunk of aragonite if it were full of capillaries like those that give granular activated carbon its high surface area. Whether this type of network of capillaries actually exist in aragonite and is accessible in part or in its entirety to phosphate remains to be demonstrated in a study such as Millero’s. An alternative explanation (just an idea, I haven't tested this in the lab) for the high adsorption rate in JDA’s experiment is the precipitation of phosphate salts during the solids addition of the very basic trisodium phosphate to artificial saltwater. What would be useful in dealing with this contradictory data is for someone to identify a source of clean highly porous aragonite that can be tested for its ability to bind phosphate. Even repeating JDA’s work with Millero’s protocol would be very interesting.
Looking forwards to your questions, comments or suggestions.
Dan
Methods. About 3 grams (measured) of washed CaribSea aragonite sand and 40 mL Instant Ocean containing phosphate were placed in a plastic 50 mL screw cap centrifuge tube and placed horizontally on an orbital shaker to gently rock the solution over the shallow bed of sand for 24 hours in the dark. The final phosphate concentration of the Instant Ocean solution was determined with the Hanna low range phosphate Checker. The difference between the initial and final concentrations corresponded to the amount of phosphate adsorbed by the sample of sand.
Desorption experiments were carried in the centrifuge tubes containing sand used in the absorption study and 40 mL 0 ppm phosphate Instant Ocean. The capped centrifuge tubes were placed horizontally on an orbital shaker for several days in the dark.
Results. Phosphate binding occurred within 6 hours but adsorption experiments were equilibrated for 24 hours to ensure the results were closer to equilibrium. The first plot below shows the amount of phosphate adsorbed at different equilibrium phosphate concentrations. The second plot shows that only a fraction of the adsorbed phosphate leaves the sand surface quickly. This is consistent with observations that removing surface bound phosphate from aragonite rocks requires a long wait.
How do the adsorption results compare to the scientific literature (Millero, 2001) and the JDA study (Post #70 for results)? The chart below summarizes what we might guesstimate about how much phosphate is bound to 20 pounds of aragonite sand equilibrated at 0.5 ppm phosphate. Because adsorption is a surface phenomenon, the amount of available surface area per unit mass needs to be known to accurately predict the bound phosphate. We do not have this number for each material in the table but we can make some educated guesses and discuss the estimates.
Millero synthesized aragonite and likely obtained a very fine precipitate. Similar synthesizes generated material with particles in the range of 50-100 microns. Materials with such a particle size in nature are classified as silt and estimated to have a surface area per gram of around 400 cm^2. The sand that I studied has particle sizes smaller than 1 mm, making it a fine to coarse sand with an estimated surface area per gram of 23-45 cm^2. With these crude estimates, the 10x range in surface area can explain much of the difference in adsorbed phosphate between my results and Millero’s. Can a surface area difference explain why large chunks of aragonite in JDA’s experiment adsorb so much more phosphate than Millero’s silty aragonite?
The simple answer is that the phosphate binding rate cannot exceed the limit observed by Millero, but lets think about this further. If the phosphate binding rate is 10X Millero’s, the surface area would have to be 10x higher or 4000 cm^2 per gram! Surface area could be larger than the visible surface area for a chunk of aragonite if it were full of capillaries like those that give granular activated carbon its high surface area. Whether this type of network of capillaries actually exist in aragonite and is accessible in part or in its entirety to phosphate remains to be demonstrated in a study such as Millero’s. An alternative explanation (just an idea, I haven't tested this in the lab) for the high adsorption rate in JDA’s experiment is the precipitation of phosphate salts during the solids addition of the very basic trisodium phosphate to artificial saltwater. What would be useful in dealing with this contradictory data is for someone to identify a source of clean highly porous aragonite that can be tested for its ability to bind phosphate. Even repeating JDA’s work with Millero’s protocol would be very interesting.
Looking forwards to your questions, comments or suggestions.
Dan



















