Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Randy's thoughts on trace elements
- Thread starter Randy Holmes-Farley
- Start date
- Tagged users None
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,586
- Reaction score
- 64,043
PO4 is around 0.05 as measured by Hanna HI774. Not sure if that relates to phosphorous in this context.
It does and that isn’t the explanation in your case.
Such high hopes for learning and understanding the chemical stew in my tank. Sigh. The nitrate / phosphate navel gazing lost me.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,586
- Reaction score
- 64,043
Such high hopes for learning and understanding the chemical stew in my tank. Sigh. The nitrate / phosphate navel gazing lost me.
Do you have questions that you would like to see addressed?
- Joined
- Aug 24, 2016
- Messages
- 1,506
- Reaction score
- 2,301
That corals can outcompete green algae on nitrogen when they have enough phosphate is backed by the fact that in intact coral reefs algae may be nitrogen limited. In fact in different reefs the nutrient ratios may differ but the fact alone that in intact coral reefs algae may be nitrogen limited says that at least some algae need more nitrogen for growth than corals, article 1, article 2, article 3, article 4."Corals will grow on the phosphate and trace elements and will outcomplete green algae at nitrogen."
This has been in almost every response I have posted.
In my own experience the nitrogen demand of algae follows the order Enteromorpha/Ulva > Bryopsis, Chaetomorpha > Valonia, Dictyosphaeria > Caulerpa, Halimeda under "normal" reef tank conditions with grazers that may prefer the softer Enteromorpha/Ulva algae.
The higher nitrogen demand of green algae can be explained by the fact that the accessory pigment chlorophyll b contains nitrogen while the caroteinoids of brown macro and micro algae do not.
Calcifying organisms (both, corals and algae) seem to have a higher demand for phosphate simply because the calcification process seems to demand phosphate and phosphates are trapped in calcarous skeletons of algae and corals.
Now to the trace elements: It was one of my first observations that nitrate and phosphate concentrations started to drop after I started dosing trace elements according to the formulas given in the link (my article) around 30 years ago. Although it is rarely mentioned I think starting trace element dosage in many tanks is the beginning of a slow phase shift from trace element limitation of coral growth and growth in reef tanks in general to a more macronutrient limited growth.
Although in Germany the worst excesses of starving a few fish to death was already over in the early 2000s, with widespread trace element dosing more tanks seemed to be macronutrient limited than before, despite much better feeding of the fish. Maybe it was also a shift in awareness but that is my conclusion on it.
Bringing both together, macronutrient limitation with trace element dosage and nitrogen limitation of green algae led me to the conclusion that with trace elements and phosphate dosage corals can outcompete green algae.
This will not work in every tank because it also depends on the feeding and the feeds (high protein vs. high phosphate) but it is possible.
Maybe, as mentioned earlier there is also a language barrier and so I may miss something that I might not have missed otherwise. If this was the case I am sorry.I think you are confusing what I’ve been asking you for with a whole lot of stuff that I haven’t said or thought.
Just for clarification: In my eyes, opinion means following an approach or a theory without trying to verify or falsify it. I think most reef aquarists do not test the approaches or theories they follow. I at least try as good as I can. And most things I have tried and tested were my own formulas, so I usually knew what I was adding for the last 30 years.
Last edited:
In the trace element article Hans-Werner linked to contains a couple of good summaries.
1 What is the biological importances of them?
Below is a figure from the article that show the use of different trace elements
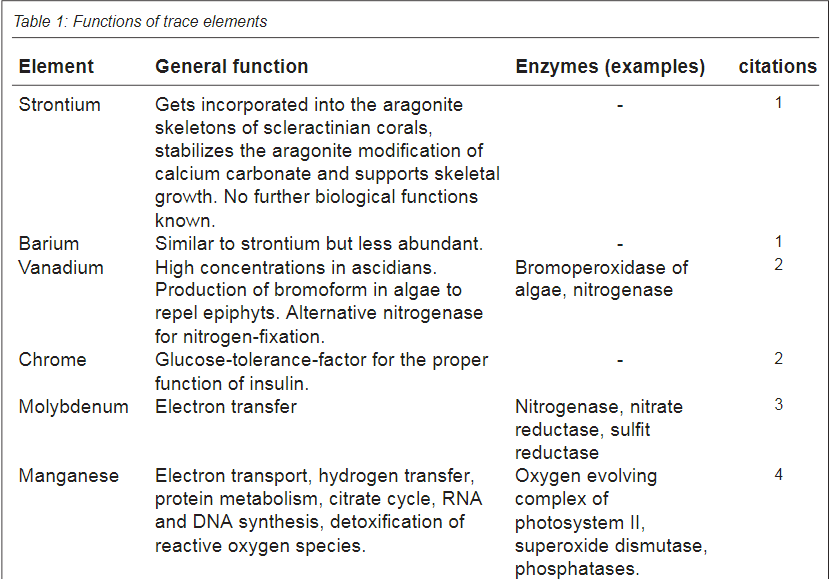
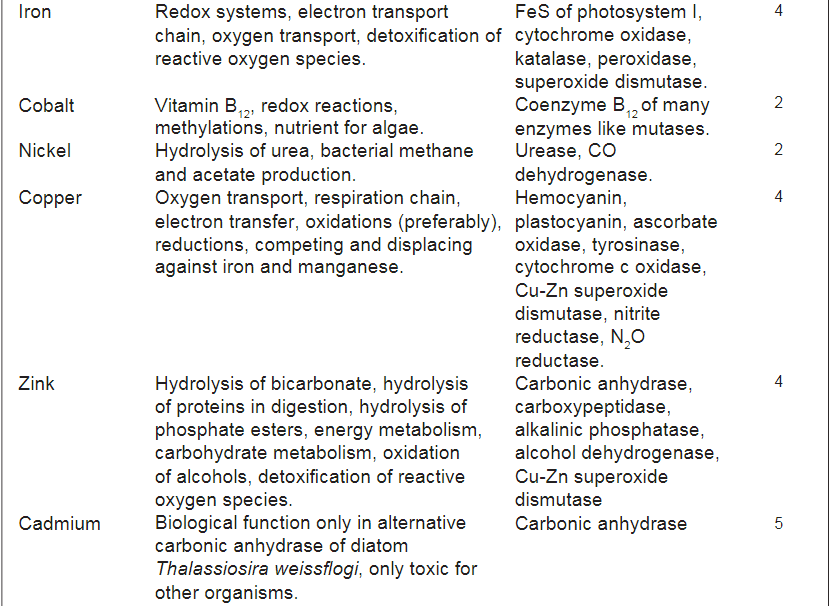

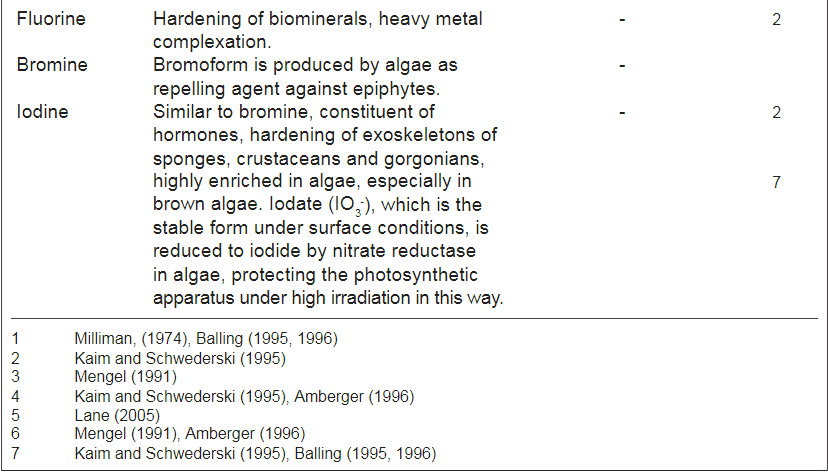
In addition to absorption in soft tissues, the table also mentions which substances are found in the calcareous skeleton of hard corals. He seems to believe that the composition of different substances in the skeleton plays a role in terms of the skeleton's resistance, hardness and other things. an opinion that I share. However, the general view seems to be that "s*h*i*t happens" just because the substances are in the water and the composition has no biological or ecological significance. IMO - if it should be a "s*h*i*t happens" event - the concentrations should - IMO - be the same in the skeleton as it is in the water - but it is not - it is biomagnified rather much in the skeleton. As an example I is between 8-6 µg/g in this table - in seawater around 0,06µg/g. Ba - between 55-116 µg/g and seawater around 0.014 µg/g
Note it is µg/g in this table
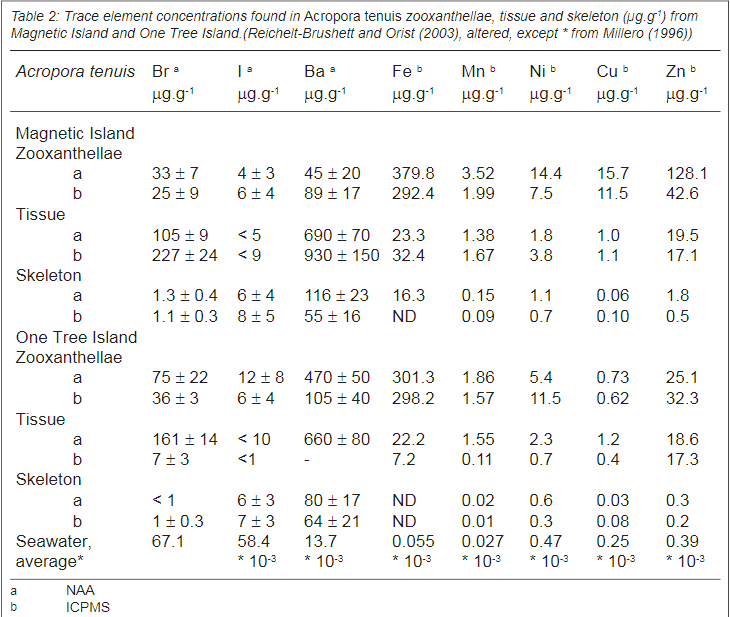
Table 3 shows this also for many other hard coral species - there is a huge biomagnification of trace elements in the skeleton compared with sea water.
Concentrations in seawater with species. Note if you want to convert mg/L to µg/g the easiest way is to see it as equal mg/L = µg/g. but in saltwater it is µg/1.024g but it has no major importance if it is 1:1 or 0.98:1
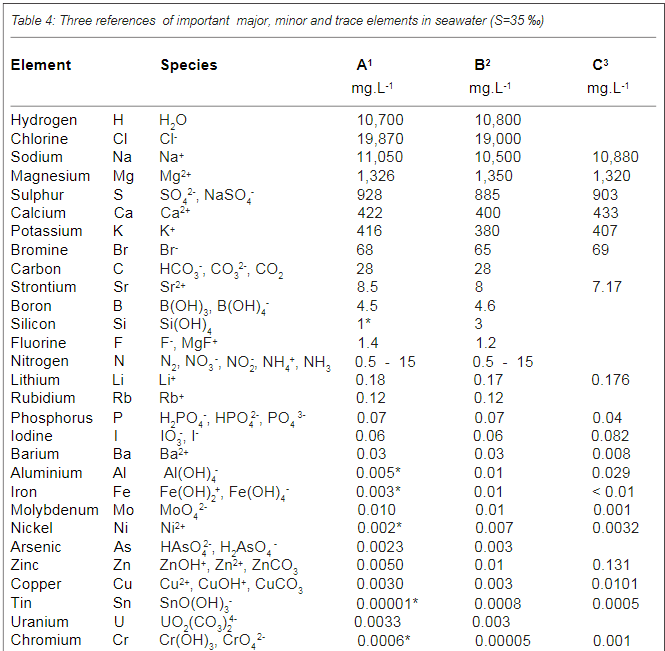
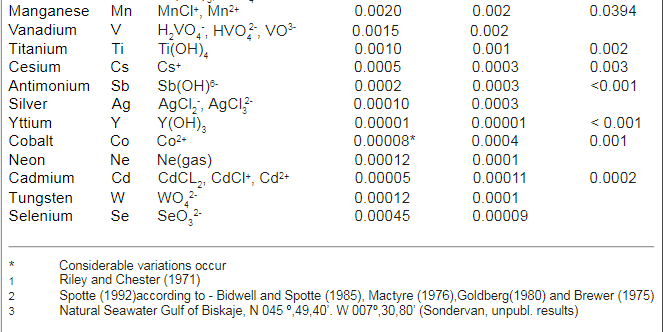
The article show some recipes of combined trace elements but this was before ICP was introduced on a broad base in the hobby - today it is easier just to follow the trend from multiple ICP analyses of the aquarium water and dose single elements - IMO
Sincerely Lasse
1 What is the biological importances of them?
Below is a figure from the article that show the use of different trace elements
In addition to absorption in soft tissues, the table also mentions which substances are found in the calcareous skeleton of hard corals. He seems to believe that the composition of different substances in the skeleton plays a role in terms of the skeleton's resistance, hardness and other things. an opinion that I share. However, the general view seems to be that "s*h*i*t happens" just because the substances are in the water and the composition has no biological or ecological significance. IMO - if it should be a "s*h*i*t happens" event - the concentrations should - IMO - be the same in the skeleton as it is in the water - but it is not - it is biomagnified rather much in the skeleton. As an example I is between 8-6 µg/g in this table - in seawater around 0,06µg/g. Ba - between 55-116 µg/g and seawater around 0.014 µg/g
Note it is µg/g in this table
Table 3 shows this also for many other hard coral species - there is a huge biomagnification of trace elements in the skeleton compared with sea water.
Concentrations in seawater with species. Note if you want to convert mg/L to µg/g the easiest way is to see it as equal mg/L = µg/g. but in saltwater it is µg/1.024g but it has no major importance if it is 1:1 or 0.98:1
The article show some recipes of combined trace elements but this was before ICP was introduced on a broad base in the hobby - today it is easier just to follow the trend from multiple ICP analyses of the aquarium water and dose single elements - IMO
Sincerely Lasse
Thanks. I am more intrested in seeing if ideas we develop or get from papers actually work in our tanks than I am in the ideas or research itself. There are too many hypotheses in our hobby that are put into husbandry practice that have no practical support, become popular, then fall out of favor because they don't really deliver predicted results, so thank you for that last sentence.This will not work in every tank because it also depends on the feeding and the feeds (high protein vs. high phosphate) but it is possible.
I agree, and I hope you will agree that it can be difficult to accept statements of action for our tanks because someone says they test the approach or theory they follow and that it is successful without showing that it has been successful.Just for clarification: In my eyes, opinion means following an approach or a theory without trying to verify or falsify it. I think most reef aquarists do not test the approaches or theories they follow. I at least try as good as I can. And most things I have tried and tested were my own formulas, so I usually knew what I was adding for the last 30 years.
- Joined
- Aug 24, 2016
- Messages
- 1,506
- Reaction score
- 2,301
Some of the elements may indeed have a role in skeletal growth and calcification. We just don't know exactly what the organic matrix of the skeleton exactly is, at least to my knowledge. Maybe some of the zinc is incorporated as active part of the enzyme carbonic anhydrase or nickel as part of the enzyme urease.
Most of the metals most likely are just precipitated as carbonates, just like calcium. The biomagnification factor of calcium is ca. 1000, from 400 mg/kg to 400.000 mg/kg (400 g/kg, 40 % in calcium carbonate). All other elements should be compared to this biomagnification factor.
However, it doesn't matter too much why they are incorporated into the skeletons, what matters is, that they a-r-e incorporated into the skeletons. This means they are out of the water, not bioavailable any more. This is one main reason why we should supplement them.
Most of the metals most likely are just precipitated as carbonates, just like calcium. The biomagnification factor of calcium is ca. 1000, from 400 mg/kg to 400.000 mg/kg (400 g/kg, 40 % in calcium carbonate). All other elements should be compared to this biomagnification factor.
However, it doesn't matter too much why they are incorporated into the skeletons, what matters is, that they a-r-e incorporated into the skeletons. This means they are out of the water, not bioavailable any more. This is one main reason why we should supplement them.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,586
- Reaction score
- 64,043
In the trace element article Hans-Werner linked to contains a couple of good summaries.
1 What is the biological importances of them?
Below is a figure from the article that show the use of different trace elements




In addition to absorption in soft tissues, the table also mentions which substances are found in the calcareous skeleton of hard corals. He seems to believe that the composition of different substances in the skeleton plays a role in terms of the skeleton's resistance, hardness and other things. an opinion that I share. However, the general view seems to be that "s*h*i*t happens" just because the substances are in the water and the composition has no biological or ecological significance. IMO - if it should be a "s*h*i*t happens" event - the concentrations should - IMO - be the same in the skeleton as it is in the water - but it is not - it is biomagnified rather much in the skeleton. As an example I is between 8-6 µg/g in this table - in seawater around 0,06µg/g. Ba - between 55-116 µg/g and seawater around 0.014 µg/g
Note it is µg/g in this table

Table 3 shows this also for many other hard coral species - there is a huge biomagnification of trace elements in the skeleton compared with sea water.
Concentrations in seawater with species. Note if you want to convert mg/L to µg/g the easiest way is to see it as equal mg/L = µg/g. but in saltwater it is µg/1.024g but it has no major importance if it is 1:1 or 0.98:1


The article show some recipes of combined trace elements but this was before ICP was introduced on a broad base in the hobby - today it is easier just to follow the trend from multiple ICP analyses of the aquarium water and dose single elements - IMO
Sincerely Lasse
I do not agree that there is sufficient evidence for some of those claims. Strontium and barium, in particular. Incorporation does not imply utility, yet folks do that anyway.
Strontium is equally incorporated into abiotic precipitation of calcium carbonate.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,586
- Reaction score
- 64,043
However, it doesn't matter too much why they are incorporated into the skeletons, what matters is, that they a-r-e incorporated into the skeletons. This means they are out of the water, not bioavailable any more. This is one main reason why we should supplement them.
So you dose uranium?
That rationale is a cover your butt reason to dose, and it is fine to do so, of course, but is not any sort of evidence that it is needed or useful.
- Joined
- Aug 24, 2016
- Messages
- 1,506
- Reaction score
- 2,301
I am talking on the essential trace elements mainly.So you dose uranium?
That rationale is a cover your butt reason to dose, and it is fine to do so, of course, but is not any sort of evidence that it is needed or useful.
The bioinorganic chemistry is on essential trace elements.
Last edited:
- Joined
- Aug 24, 2016
- Messages
- 1,506
- Reaction score
- 2,301
Yes, I agree, but want to differ it a bit: Not all published ideas are usefull for us, but without a good explanation anectodic "evidence" is weak. It gets much stronger if there is some scientific backing.Thanks. I am more intrested in seeing if ideas we develop or get from papers actually work in our tanks than I am in the ideas or research itself. There are too many hypotheses in our hobby that are put into husbandry practice that have no practical support, become popular, then fall out of favor because they don't really deliver predicted results, so thank you for that last sentence.
You may even follow a wrong track and find it excluded by science and led onto a better track that is supported by science. This in my eyes are the practical applications of scientific findings.
Absolutely. The thing that I am on the lookout for is aquarists jumping to action before or without practical support, and then presenting that action as something everyone should do. Discussion, and testing, and trying, and experimenting is great, but all too often in this hobby people are recommending actions before that has been done, or without any compelling evidence that it actually works.Yes, I agree, but want to differ it a bit: Not all published ideas are usefull for us, but without a good explanation anectodic "evidence" is weak. It gets much stronger if there is some scientific backing.
You may even follow a wrong track and find it excluded by science and led onto a better track that is supported by science. This in my eyes are the practical applications of scientific findings.
The far reaching discussion about what might be happening is great and needed and wonderful. I just see too many hobbyists having a bad time because they are chasing 'methods' that aren't practically supported.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,586
- Reaction score
- 64,043
. IMO - if it should be a "s*h*i*t happens" event - the concentrations should - IMO - be the same in the skeleton as it is in the water - but it is not - it is biomagnified rather much in the skeleton. As an example I is between 8-6 µg/g in this table - in seawater around 0,06µg/g. Ba - between 55-116 µg/g and seawater around 0.014 µg/g
All utility issues aside, that assertion does not make chemical sense.
If there is no biological mechanism adding or excluding ions from a skeleton, they absolutely will not get into it by mistake in the same ratio as the concentrations of them in seawater.
The higher the concentration of a single ion, the more will get in by abiotic incorporation, but how much actually gets in for a particular ion at a given concentration depends critically on the free energy of incorporation into the mineral of interest. Some ions fit pretty well into aragonite, and others not so well.
Magnesium and strontium are incorporated into deposited aragonite, even abiotic aragonite, in ratios that do not reflect the seawater concentration.
Your suggestion would make it impossible for oolitic aragonite to ever form since magnesium is always present at far higher concentration than calcium, and yet is incorporated into the abiotic mineral at pretty low levels.
I just wanted to weigh in on the accuracy of testing (or lack-there-of) regarding trace elements.
I know this has been brought up in here in a few posts prior but I recently conducted a "free trial" ICP and the results that came back were largely inaccurate based of MY OWN personal testing. Obviously there is ample opportunity for error on both ends...This is just my own personal anecdote.
1) Calcium was reported at 354. My tests verify a number somewhere between 425-480. Checked against a reference solution.
2) Salinity was reported at 28 ppt. My tests verify my water at 33-35ppt consistently, refractometer calibrated weekly with two purchased solutions and Randy's homemade calibration solution.
3) Magnesium was reported at 1184 (I understand there is often test error/inaccuracy here) - My tests verify between 1340-1390 checked against a reference solution.
I had several trace elements that were reported as "low" and included strontium, zinc, iron, and several others.
Point being, if there is so much discrepancy between my tests and an ICP analysis, how can we be at all sure that we NEED to supplement in the first place. Especially minor elements that are not entirely understood in their respective roles regarding biological processes.
I know some folks report evidence that the introduction of supplemental dosing programs have done wonders. Great. On the other side of the coin, there are scenarios where we see thriving reef tanks without supplementation or consistent analysis of trace elements. Also great.
IMO, I do not have enough evidence that supports the idea that trace supplementation is absolutely necessary. Industry standards in shipping/handling of samples opens the door to multiple variables that may skew actual results from these tests, leading me to doubt pinpoint accuracy in the first place. Some of my "low" numbers were just under the "recommended standard", who sets these limits? Is it solely based of concentration found within NSW? Again, these are element that are not completely understood and how they fill a role in biological processes.
Makes my brain hurt a little lol..
I know this has been brought up in here in a few posts prior but I recently conducted a "free trial" ICP and the results that came back were largely inaccurate based of MY OWN personal testing. Obviously there is ample opportunity for error on both ends...This is just my own personal anecdote.
1) Calcium was reported at 354. My tests verify a number somewhere between 425-480. Checked against a reference solution.
2) Salinity was reported at 28 ppt. My tests verify my water at 33-35ppt consistently, refractometer calibrated weekly with two purchased solutions and Randy's homemade calibration solution.
3) Magnesium was reported at 1184 (I understand there is often test error/inaccuracy here) - My tests verify between 1340-1390 checked against a reference solution.
I had several trace elements that were reported as "low" and included strontium, zinc, iron, and several others.
Point being, if there is so much discrepancy between my tests and an ICP analysis, how can we be at all sure that we NEED to supplement in the first place. Especially minor elements that are not entirely understood in their respective roles regarding biological processes.
I know some folks report evidence that the introduction of supplemental dosing programs have done wonders. Great. On the other side of the coin, there are scenarios where we see thriving reef tanks without supplementation or consistent analysis of trace elements. Also great.
IMO, I do not have enough evidence that supports the idea that trace supplementation is absolutely necessary. Industry standards in shipping/handling of samples opens the door to multiple variables that may skew actual results from these tests, leading me to doubt pinpoint accuracy in the first place. Some of my "low" numbers were just under the "recommended standard", who sets these limits? Is it solely based of concentration found within NSW? Again, these are element that are not completely understood and how they fill a role in biological processes.
Makes my brain hurt a little lol..
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,586
- Reaction score
- 64,043
I just wanted to weigh in on the accuracy of testing (or lack-there-of) regarding trace elements.
I know this has been brought up in here in a few posts prior but I recently conducted a "free trial" ICP and the results that came back were largely inaccurate based of MY OWN personal testing. Obviously there is ample opportunity for error on both ends...This is just my own personal anecdote.
1) Calcium was reported at 354. My tests verify a number somewhere between 425-480. Checked against a reference solution.
2) Salinity was reported at 28 ppt. My tests verify my water at 33-35ppt consistently, refractometer calibrated weekly with two purchased solutions and Randy's homemade calibration solution.
3) Magnesium was reported at 1184 (I understand there is often test error/inaccuracy here) - My tests verify between 1340-1390 checked against a reference solution.
Any chance it froze?

Warning about ICP procedures that can cause false readings: freezing
I do not know what tube filling procedures are advised by all ICP companies, but a recent test by Christoph at Oceamo points out a potentially serious concern. Freezing of seawater causes the formation of fresh water ice and hypersaline seawater. As the ice spreads to more and more of the...
 www.reef2reef.com
www.reef2reef.com
And this is why I’m not sure so many people follow reef moonshiners program and buy all that crap! lolI just wanted to weigh in on the accuracy of testing (or lack-there-of) regarding trace elements.
I know this has been brought up in here in a few posts prior but I recently conducted a "free trial" ICP and the results that came back were largely inaccurate based of MY OWN personal testing. Obviously there is ample opportunity for error on both ends...This is just my own personal anecdote.
1) Calcium was reported at 354. My tests verify a number somewhere between 425-480. Checked against a reference solution.
2) Salinity was reported at 28 ppt. My tests verify my water at 33-35ppt consistently, refractometer calibrated weekly with two purchased solutions and Randy's homemade calibration solution.
3) Magnesium was reported at 1184 (I understand there is often test error/inaccuracy here) - My tests verify between 1340-1390 checked against a reference solution.
I had several trace elements that were reported as "low" and included strontium, zinc, iron, and several others.
Point being, if there is so much discrepancy between my tests and an ICP analysis, how can we be at all sure that we NEED to supplement in the first place. Especially minor elements that are not entirely understood in their respective roles regarding biological processes.
I know some folks report evidence that the introduction of supplemental dosing programs have done wonders. Great. On the other side of the coin, there are scenarios where we see thriving reef tanks without supplementation or consistent analysis of trace elements. Also great.
IMO, I do not have enough evidence that supports the idea that trace supplementation is absolutely necessary. Industry standards in shipping/handling of samples opens the door to multiple variables that may skew actual results from these tests, leading me to doubt pinpoint accuracy in the first place. Some of my "low" numbers were just under the "recommended standard", who sets these limits? Is it solely based of concentration found within NSW? Again, these are element that are not completely understood and how they fill a role in biological processes.
Makes my brain hurt a little lol..
Seems some of these programs regardless of topic feed off cult tendencies. I’m studying it but not one to go down to mad scientist status.And this is why I’m not sure so many people follow reef moonshiners program and buy all that crap! lol
Hmmmm….. I do look at it another way. People experiment and in year or two or three there will be enough feedback to see if the system works or maybe works or doesn’t work. It is a hobby so experimenting is part of the package and money might not be spent wisely.And this is why I’m not sure so many people follow reef moonshiners program and buy all that crap! lol
When I started there was the ULNS following with zero PO4/ NO3. That is what I tried and I was real good at growing dinos etc… and no corals. To some extent it is still around but I think the consensus now is the reef tank needs some PO4/P and some NO3/N.
I'm assuming it must have. Went to CO.Any chance it froze?

Warning about ICP procedures that can cause false readings: freezing
I do not know what tube filling procedures are advised by all ICP companies, but a recent test by Christoph at Oceamo points out a potentially serious concern. Freezing of seawater causes the formation of fresh water ice and hypersaline seawater. As the ice spreads to more and more of the...www.reef2reef.com
Point and case, without a standard in shipping/handling we can't be sure that our samples are received in good condition. Not pointing fingers at the companies conducting the tests - but I think the amount of variance is something that needs to be accounted for and should be considered before individuals react.
I won't be changing my routine or buying up additives needless to say.
I think these testing procedures can be helpful when certain protocols are established and coupled with definitive research that provides supporting evidence that trace element supplementation is necessary. Until then, I'm out.
Last edited:
Similar threads
-
- AMS: Article
- Replies
- 96
- Views
- 6,271
- Replies
- 14
- Views
- 679
- Replies
- 5
- Views
- 207
- Replies
- 7
- Views
- 251
- Replies
- 9
- Views
- 203











