I mean the pH meter says 7.6. It's in a molecular lab so it gets calibrated very often. Unless there is something in the water (in the vial) producing CO2, I have no clue how that could be. I open the windows every day for at least 20 minutes so I doubt co2 could be at harmful levels.A pH of 7.6 is not a realistic measurement unless your tank aeration is poor or your adding low-pH additives.
Your indoor CO2 would be at unhealthy levels that would cause you health issues with prolonged exposure.
If you believe your pH is 7.6, then you need to get more water movement on the tank.
A simple cup aeration test can confirm your issue.
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Tested pH with lab pH meter and it's 7.5!
- Thread starter KonradTO
- Start date
- Tagged users None
I mean the pH meter says 7.6. It's in a molecular lab so it gets calibrated very often. Unless there is something in the water (in the vial) producing CO2, I have no clue how that could be. I open the windows every day for at least 20 minutes so I doubt co2 could be at harmful levels.
If you are 100% confident in your PH AND Alk readings then it really leaves you with the scenario where you need more gas exchange in the tank(or you have extremely high co2 levels)
Personally as I stated earlier in the thread I still think the only reasonable first step in these scenarios is an outdoor and indoor aeration test. Otherwise it is just all about people guessing, but it boils down to alkalinity and co2 in the tank (accuracy of testing is always a possible variable). The aeration tests may not give you the exact answer but they can rule out things and put you on the right track.
What scale is the pH probe reading in? Are you aware there are different scales?
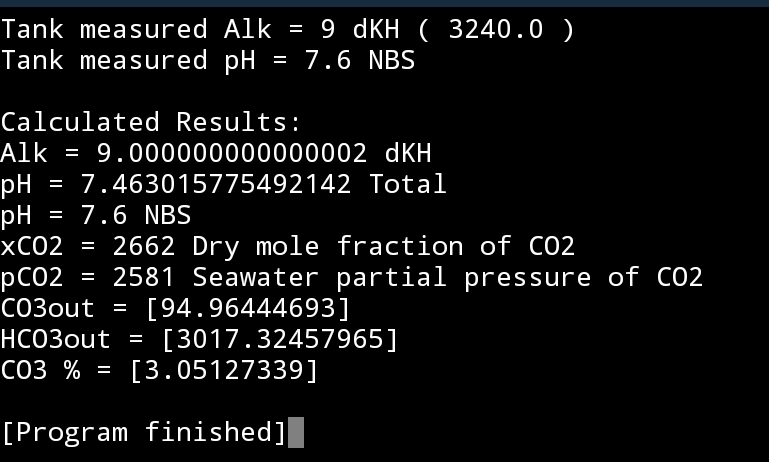
7.6 total ~ 7.73 NBSWhat scale is the pH probe reading in? Are you aware there are different scales?
Which would still put your indoor CO2 at 1900ppm or at a very unhealthy level that would cause health issues with longer exposure.
I got finally the portable air pump, I will bring my water sample tomorrow to the lab and repeat. I am starting to doubt my kh test now.. I have loads of macro in my tank I cannot immagine they would not deal with dissolved co2, I have plenty of surface agitation and I definitely do not have problems with co2 levels in the house since I have been living here for 3 years now and every winter it's as cold as the previousIf you are 100% confident in your PH AND Alk readings then it really leaves you with the scenario where you need more gas exchange in the tank(or you have extremely high co2 levels)
Personally as I stated earlier in the thread I still think the only reasonable first step in these scenarios is an outdoor and indoor aeration test. Otherwise it is just all about people guessing, but it boils down to alkalinity and co2 in the tank (accuracy of testing is always a possible variable). The aeration tests may not give you the exact answer but they can rule out things and put you on the right track.
I did the aeration test today. Measured pH, then run the air stone for 1h outside and re-measured pH.If you are 100% confident in your PH AND Alk readings then it really leaves you with the scenario where you need more gas exchange in the tank(or you have extremely high co2 levels)
Personally as I stated earlier in the thread I still think the only reasonable first step in these scenarios is an outdoor and indoor aeration test. Otherwise it is just all about people guessing, but it boils down to alkalinity and co2 in the tank (accuracy of testing is always a possible variable). The aeration tests may not give you the exact answer but they can rule out things and put you on the right track.
Before aeration was 7.63
After aeration 7.75
How should I interpret this?
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,311
- Reaction score
- 63,661
I did the aeration test today. Measured pH, then run the air stone for 1h outside and re-measured pH.
Before aeration was 7.63
After aeration 7.75
How should I interpret this?
Closing up a sample and transporting it for pH can be fraught with issues relating to production of CO2 in the closed sample.
Was this sample similarly transported, or measured right away after aeration?
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,311
- Reaction score
- 63,661
Also I have only 3 small fishes and few inverts, I am quite sure the co2 they produce is taken up by my caulerpa
macroalgae typically do not solve low pH issues driven by CO2 in the home air.
No I aerated directly at work. I left it 45 minutes sealed at room temperature after aeration because was very cold.Closing up a sample and transporting it for pH can be fraught with issues relating to production of CO2 in the closed sample.
Was this sample similarly transported, or measured right away after aeration?
May I ask why not? There is no difference from CO2 in home air and CO2 created as a result of biological processes in the tank..macroalgae typically do not solve low pH issues driven by CO2 in the home air.
I guess he means that their CO2 uptake isn't fast enough to counteract the acidification caused by high CO2 in the house..May I ask why not? There is no difference from CO2 in home air and CO2 created as a result of biological processes in the tank..
The point is that I had only a 0.1 difference in pH after aeration.. Does that mean that indoor CO2 is not a problem or a 0.1 change in 1h means that the problem is actually CO2?
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,311
- Reaction score
- 63,661
May I ask why not? There is no difference from CO2 in home air and CO2 created as a result of biological processes in the tank..
Of course I'm not saying there's a difference. My point was that the posters assertion (posted below) has a false premise: that CO2 cannot be high because macroalgae would take it all up.
" I have only 3 small fishes and few inverts, I am quite sure the co2 they produce is taken up by my caulerpa"
I'm saying the magnitude of effect from growing macroalgae is not typically sufficient to eliminate a high CO2 problem caused by high CO2 home air.
That's a simple recounting of the results people attain when trying various ways to reduce CO2.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,311
- Reaction score
- 63,661
The point is that I had only a 0.1 difference in pH after aeration.. Does that mean that indoor CO2 is not a problem or a 0.1 change in 1h means that the problem is actually CO2?
It suggests to me that the pH measurement is off, or the air you aerated with has high CO2.
It suggests to me that the pH measurement is off, or the air you aerated with has high CO2.
If the aeration was done with outdoor air and your alkalinity is above 7, the pH should minimally be 8.2 NBS.
Understood - thank you, Randy.Of course I'm not saying there's a difference. My point was that the posters assertion (posted below) has a false premise: that CO2 cannot be high because macroalgae would take it all up.
" I have only 3 small fishes and few inverts, I am quite sure the co2 they produce is taken up by my caulerpa"
I'm saying the magnitude of effect from growing macroalgae is not typically sufficient to eliminate a high CO2 problem caused by high CO2 home air.
That's a simple recounting of the results people attain when trying various ways to reduce CO2.
I will bring to my lfs a sample of water for doublecheking the kh, since I am 100 % sure about pH. And yes, obviously aeration test was performed outsideIf the aeration was done with outdoor air and your alkalinity is above 7, the pH should minimally be 8.2 NBS.
Similar threads
- Replies
- 6
- Views
- 125
- Replies
- 27
- Views
- 367
- Replies
- 8
- Views
- 251
- Replies
- 5
- Views
- 103


















