- Joined
- Sep 21, 2018
- Messages
- 6,675
- Reaction score
- 7,170
Not too long ago I made a claim about needing less water to wash new aragonite sand if you first sieve it to remove the fines.
https://www.reef2reef.com/threads/quit-wasting-water-washing-dry-aragonite-sand.953390/
Long story short, I was wrong. So, I went back to the lab to understand aragonite sand washing. Here is the new data that gives us insight into why aragonite sand is nearly impossible to rinse clean.
Everyone who has ever tried to wash aragonite sand until the wash water ran clear, faces performing what seems like an infinite number of washes. The orange line on the plot below shows how the cloudiness of the wash water (measured with the Hanna Color of Water Checker) declines rapidly at first but then refuses to budge much lower.
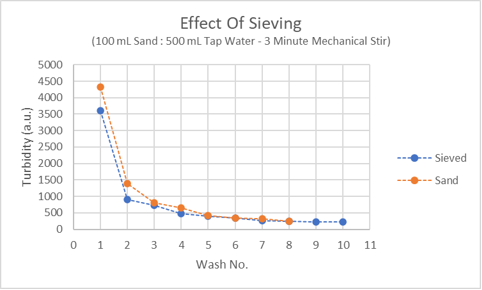
I initially thought that removing the fines by sieving the sand first would solve the problem of “infinite washes”. The blue line in the above plot shows it does not help. What is going on?
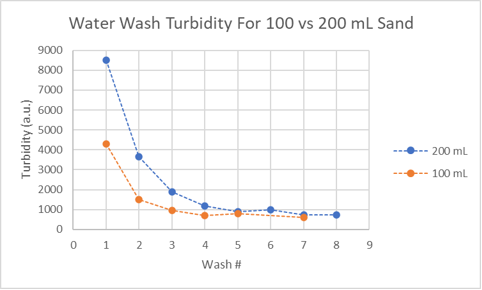
One of the first hints about what is happening during aragonite sand washing came from comparing data from two sand washing experiments. By overlaying the turbidity data from washing 100 mL and 200 mL of sand, the process looks like it might involve two stages. The first stage is where the turbidity is correlated with the initial sand mass while the second stage seems to occur with little or no difference between the two initial sand masses. The correlation of the first stage turbidity with the initial sand mass suggests turbidity is relates to the amount of fines suspended in the wash water, while the second stage turbidity, seemingly unrelated to the amount of sand present, means what?
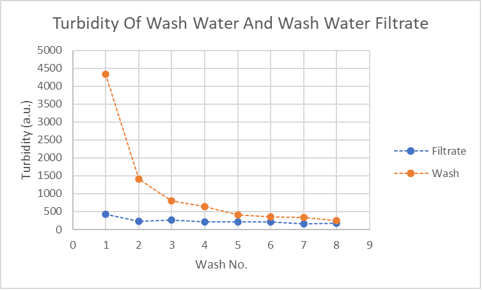
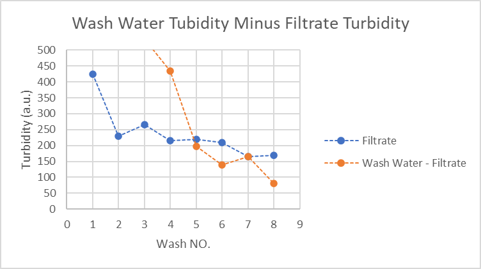
To investigate the process in the second stage further and determine whether it occurs throughout the entire sand washing exercise, the turbidity of the wash water was measured, then it was filtered through a 1.6 micron glass filter to remove the fines. Each filter was weighed before use and then again after filtration and drying. Turbidity of the filtrate was also measured to assess how much turbidity there was that was not associated with the fines in the sand. Comparing the turbidities of the wash water and filtrate (see plot) demonstrates that the second turbidity generating process occurs in every wash.
Comparing turbidities of the filtrate against the wash water turbidity minus the filtrate turbidity indicates that the decline in turbidity caused by fines might be faster (orange line) than the decline in turbidity caused by the second stage process (blue line).
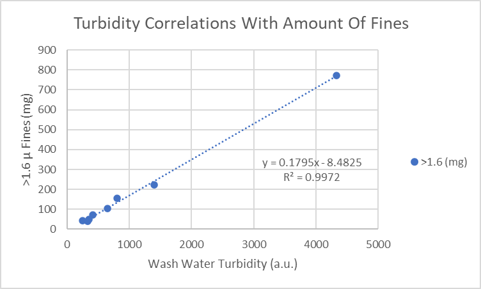
With regards to the mass of the filtered fines, it is well correlated to the turbidity of the wash water (see plot). The intercept is not zero pribably because of the turbidity caused by stage two turbidity.
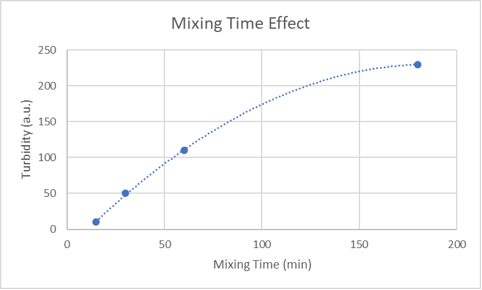
Data that gave me a hint about what might be happening in the second turbidity generating process involved stirring washed sand in tap water for varying lengths of time (see plot below). This data makes clear that wash water turbidity rapidly increases with the time that sand is exposed to tap water. Interestingly, stirring aragonite sand for one hour in tap water resulted in wash water resembling a thin, translucent white paint. This last observation suggests that a crystallization or precipitation is occurring. But why?
The sand used in the experiments contains aragonite, a form of calcium carbonate. Calcium carbonate has two major polymorphs or crystal forms, aragonite and calcite. Aragonite is typically formed by living organisms and is less stable than calcite, just like diamond is less stable than graphite. A manifestation of calcite stability is its lower solubility in water compared to aragonite. The consequence is that when aragonite dissolves in water (calcium carbonate is slightly soluble in freshwater), the concentration of calcium carbonate exceeds the solubility limit of calcite and crystals form. This dissolution-crystallization process can continue as long as there is aragonite present because crystallization continually removes calcium carbonate from solution, allowing more aragonite to dissolve. Stirring aragonite sand for an hour created a large amount of microscopic crystals that formed an emulsion-like mixture resembling thin paint. Can this process be inhibited?
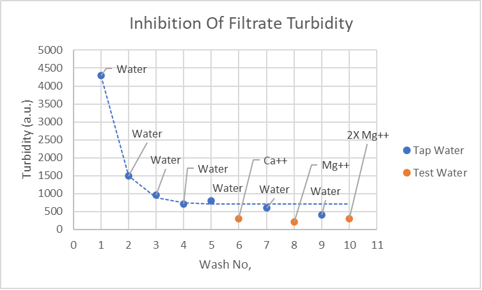
By using 1000 ppm Ca++ (common ion effect) or 1000 ppm Mg++ (calcium carbonate crystallization inhibition) in tap water, turbidity can be reduced but not eliminated (see plot). Twenty percent isopropanol in tap water also diminishes turbidity to a similar extent.
To demonstrate that minimizing aragonite sand exposure is also beneficial for large scale sand washing, six kg of aragonite sand was washed eight times with ten liters of tap water each wash. I used a power drill equipped with a paint mixer to thoroughly mix the sand in a minimum amount of time. I discussed the power drill here

 www.reef2reef.com
www.reef2reef.com
Mix time was 10 seconds. The trend in turbidity for the large scale washes was similar to that observed for the small scale pilot run (see plot).
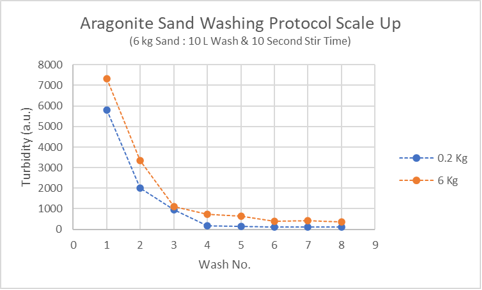
Samples of the wash water (photo below) show how turbidity caused by fines is quickly reduced and how persistently the wash water remains cloudy. Water is likely being wasted after the fourth wash.

https://www.reef2reef.com/threads/quit-wasting-water-washing-dry-aragonite-sand.953390/
Long story short, I was wrong. So, I went back to the lab to understand aragonite sand washing. Here is the new data that gives us insight into why aragonite sand is nearly impossible to rinse clean.
Everyone who has ever tried to wash aragonite sand until the wash water ran clear, faces performing what seems like an infinite number of washes. The orange line on the plot below shows how the cloudiness of the wash water (measured with the Hanna Color of Water Checker) declines rapidly at first but then refuses to budge much lower.
I initially thought that removing the fines by sieving the sand first would solve the problem of “infinite washes”. The blue line in the above plot shows it does not help. What is going on?
One of the first hints about what is happening during aragonite sand washing came from comparing data from two sand washing experiments. By overlaying the turbidity data from washing 100 mL and 200 mL of sand, the process looks like it might involve two stages. The first stage is where the turbidity is correlated with the initial sand mass while the second stage seems to occur with little or no difference between the two initial sand masses. The correlation of the first stage turbidity with the initial sand mass suggests turbidity is relates to the amount of fines suspended in the wash water, while the second stage turbidity, seemingly unrelated to the amount of sand present, means what?
To investigate the process in the second stage further and determine whether it occurs throughout the entire sand washing exercise, the turbidity of the wash water was measured, then it was filtered through a 1.6 micron glass filter to remove the fines. Each filter was weighed before use and then again after filtration and drying. Turbidity of the filtrate was also measured to assess how much turbidity there was that was not associated with the fines in the sand. Comparing the turbidities of the wash water and filtrate (see plot) demonstrates that the second turbidity generating process occurs in every wash.
Comparing turbidities of the filtrate against the wash water turbidity minus the filtrate turbidity indicates that the decline in turbidity caused by fines might be faster (orange line) than the decline in turbidity caused by the second stage process (blue line).
With regards to the mass of the filtered fines, it is well correlated to the turbidity of the wash water (see plot). The intercept is not zero pribably because of the turbidity caused by stage two turbidity.
Data that gave me a hint about what might be happening in the second turbidity generating process involved stirring washed sand in tap water for varying lengths of time (see plot below). This data makes clear that wash water turbidity rapidly increases with the time that sand is exposed to tap water. Interestingly, stirring aragonite sand for one hour in tap water resulted in wash water resembling a thin, translucent white paint. This last observation suggests that a crystallization or precipitation is occurring. But why?
The sand used in the experiments contains aragonite, a form of calcium carbonate. Calcium carbonate has two major polymorphs or crystal forms, aragonite and calcite. Aragonite is typically formed by living organisms and is less stable than calcite, just like diamond is less stable than graphite. A manifestation of calcite stability is its lower solubility in water compared to aragonite. The consequence is that when aragonite dissolves in water (calcium carbonate is slightly soluble in freshwater), the concentration of calcium carbonate exceeds the solubility limit of calcite and crystals form. This dissolution-crystallization process can continue as long as there is aragonite present because crystallization continually removes calcium carbonate from solution, allowing more aragonite to dissolve. Stirring aragonite sand for an hour created a large amount of microscopic crystals that formed an emulsion-like mixture resembling thin paint. Can this process be inhibited?
By using 1000 ppm Ca++ (common ion effect) or 1000 ppm Mg++ (calcium carbonate crystallization inhibition) in tap water, turbidity can be reduced but not eliminated (see plot). Twenty percent isopropanol in tap water also diminishes turbidity to a similar extent.
To demonstrate that minimizing aragonite sand exposure is also beneficial for large scale sand washing, six kg of aragonite sand was washed eight times with ten liters of tap water each wash. I used a power drill equipped with a paint mixer to thoroughly mix the sand in a minimum amount of time. I discussed the power drill here

How To Rinse New Sand With Less Water
Recently, I spent time in the lab looking at why so much water is needed to rinse the fines from new dry aragonite sand (Caribsea). I blame @brandon429 for such an odd pursuit. Fines are the very small bits of sand that can cloud aquarium water for days when they are not rinsed from the sand...
 www.reef2reef.com
www.reef2reef.com
Mix time was 10 seconds. The trend in turbidity for the large scale washes was similar to that observed for the small scale pilot run (see plot).
Samples of the wash water (photo below) show how turbidity caused by fines is quickly reduced and how persistently the wash water remains cloudy. Water is likely being wasted after the fourth wash.