- Joined
- Jul 28, 2015
- Messages
- 4,668
- Reaction score
- 3,191
Do you use Kalk/limewater for your ATO? Alk swings could be happening as well due to more evaporation as humidity is driven down here in the winter. I would guess lighting is most of it. But it could very well be a combination of many factors which each alone would look just fine.
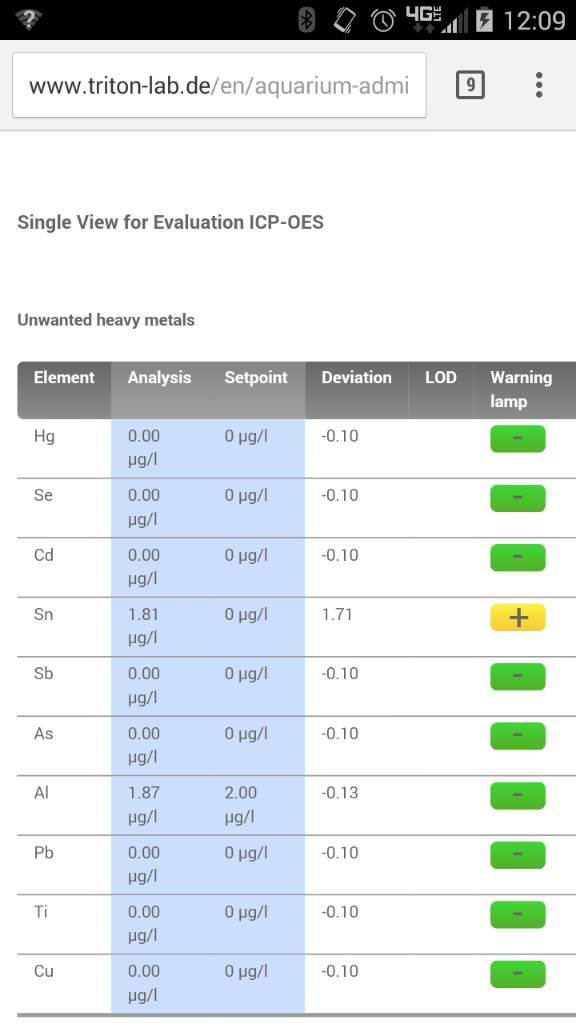
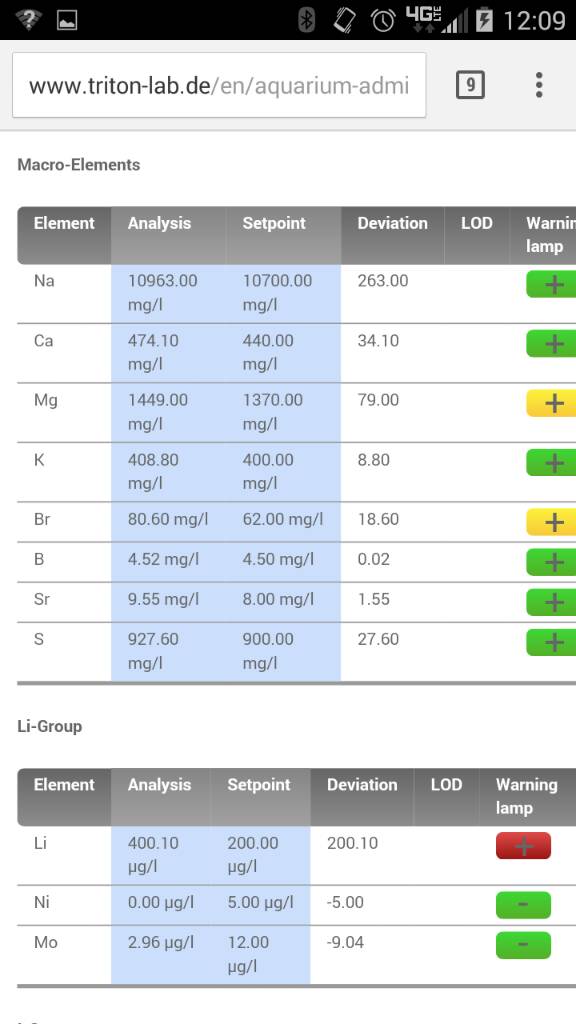
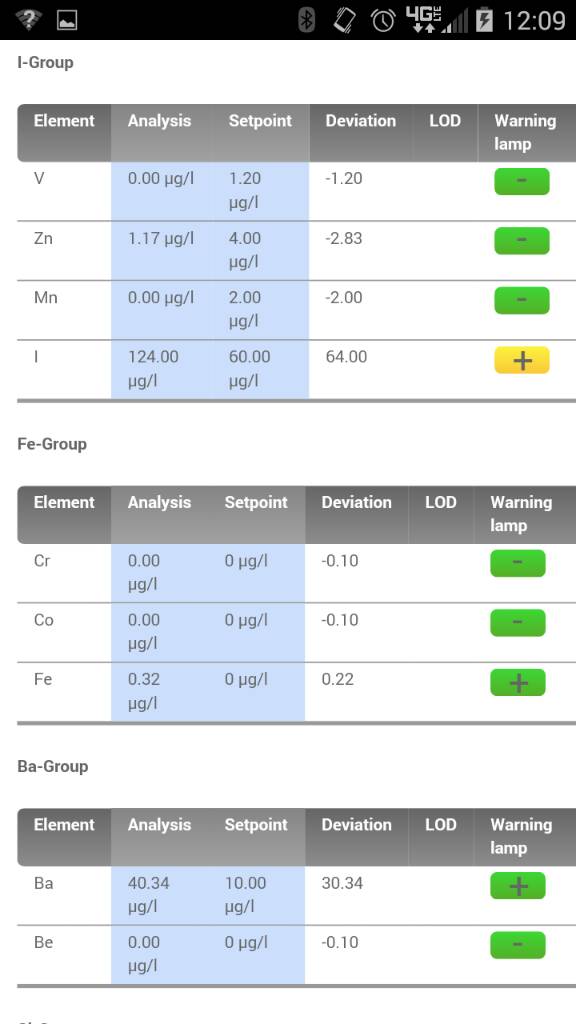
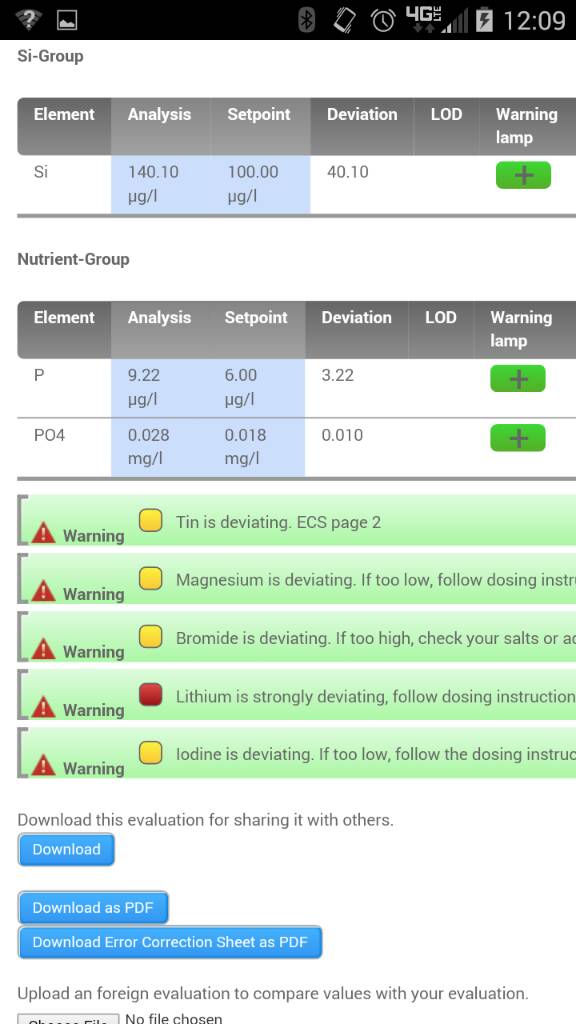
But lighting is critical and SPS do not like alk swings. Or higher alk when nutrients like NO3 and PO4 are very very low which at 8dkh if accurate I would assume is fine.
But lighting is critical and SPS do not like alk swings. Or higher alk when nutrients like NO3 and PO4 are very very low which at 8dkh if accurate I would assume is fine.