I borrowed my old PARwise to make measurements of the UV lamp outout and merged those measurements with some blue LEDs I have laying around. PARwise didn't fare so well.
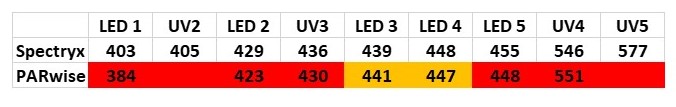
Based on the measurements I mada using the Spectryx against a white fluorescent bulb, and against a UV sterilization lamp, I'm comfident in my Spectryx's accuracy between 400 and 700 nanometers. Using the Spectryx as a validated reference, you can see the differences between the reference and PARwise. As you go from 440nm toward UV, PARwise reads increasingly lower wavelengths. Above 448nm, it can't be trusted at all, apparently.
The second issue with PARwise is that when measuring the UV lamp it couldn't identify any UV peaks as narrow band, or even detect one at all (405nm). Spectryx was definitely able to identify 4 of them precisely.
The table shows readings of single LEDs and 4 UV peaks from the UV lamp. Yellow cells identify the measurements that were close to the reference. Consider the Spectrix readings as "truth", or close to it.
So in my personal experience and opinion, PARwise has no value when it comes to measuring spectrum. If you remove the pretty colors it's fairly obvious.
Based on the measurements I mada using the Spectryx against a white fluorescent bulb, and against a UV sterilization lamp, I'm comfident in my Spectryx's accuracy between 400 and 700 nanometers. Using the Spectryx as a validated reference, you can see the differences between the reference and PARwise. As you go from 440nm toward UV, PARwise reads increasingly lower wavelengths. Above 448nm, it can't be trusted at all, apparently.
The second issue with PARwise is that when measuring the UV lamp it couldn't identify any UV peaks as narrow band, or even detect one at all (405nm). Spectryx was definitely able to identify 4 of them precisely.
The table shows readings of single LEDs and 4 UV peaks from the UV lamp. Yellow cells identify the measurements that were close to the reference. Consider the Spectrix readings as "truth", or close to it.
So in my personal experience and opinion, PARwise has no value when it comes to measuring spectrum. If you remove the pretty colors it's fairly obvious.
Last edited: