I recently switched to IO salt based on Randy's observations about various salt mixes. However, with the two bags of salt that I have purchased (50G mix size) I have encountered what I think is fairly large variation in the two on freshly mixed batches. I mix up 5 gallons at a time. In order to get 35 ppt salinity I've determined I need to add 770g of salt. A powerhead mixes the saltwater for 24 hours before I test it. Here are the results I've seen:
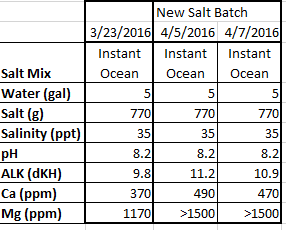
The ALK is higher than I expected. Not sure what is standard deviation for salt mix. The big differences in Ca and Mg is what concerns me the most.
I've done searches on forums to see what is expected, but all I've seen is anecdotal statements like "big variations" or "never had any issues". Does anyone know if this is typical?
Thanks for any help. (BTW- I would like a salt mix that gives ALK~8, Ca~420, & Mg~1300. If there is a better option than IO, I'm open to suggestions.)
The ALK is higher than I expected. Not sure what is standard deviation for salt mix. The big differences in Ca and Mg is what concerns me the most.
I've done searches on forums to see what is expected, but all I've seen is anecdotal statements like "big variations" or "never had any issues". Does anyone know if this is typical?
Thanks for any help. (BTW- I would like a salt mix that gives ALK~8, Ca~420, & Mg~1300. If there is a better option than IO, I'm open to suggestions.)


















