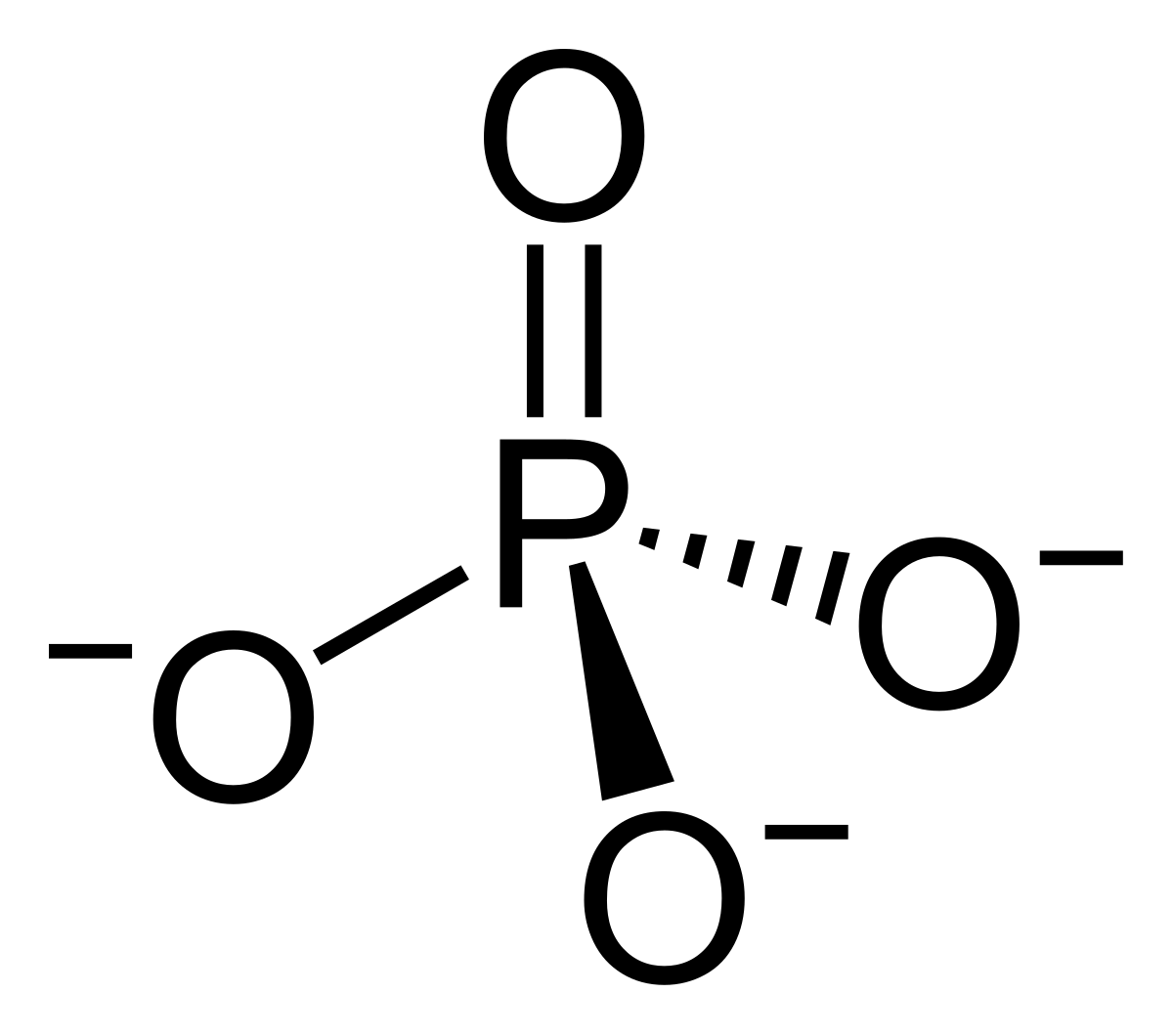
Which molecule is on Randy Holmes-Farley avatar?
a) Ammonium cation
b) Phosphate anion
c) Sulfate anion
d) those are just lollipops
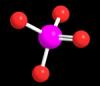
And please elaborate why. (I mean why do you think this is correct answer, not why Randy chose this avatar
a) Ammonium cation
b) Phosphate anion
c) Sulfate anion
d) those are just lollipops
And please elaborate why. (I mean why do you think this is correct answer, not why Randy chose this avatar