Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,516
- Reaction score
- 63,967
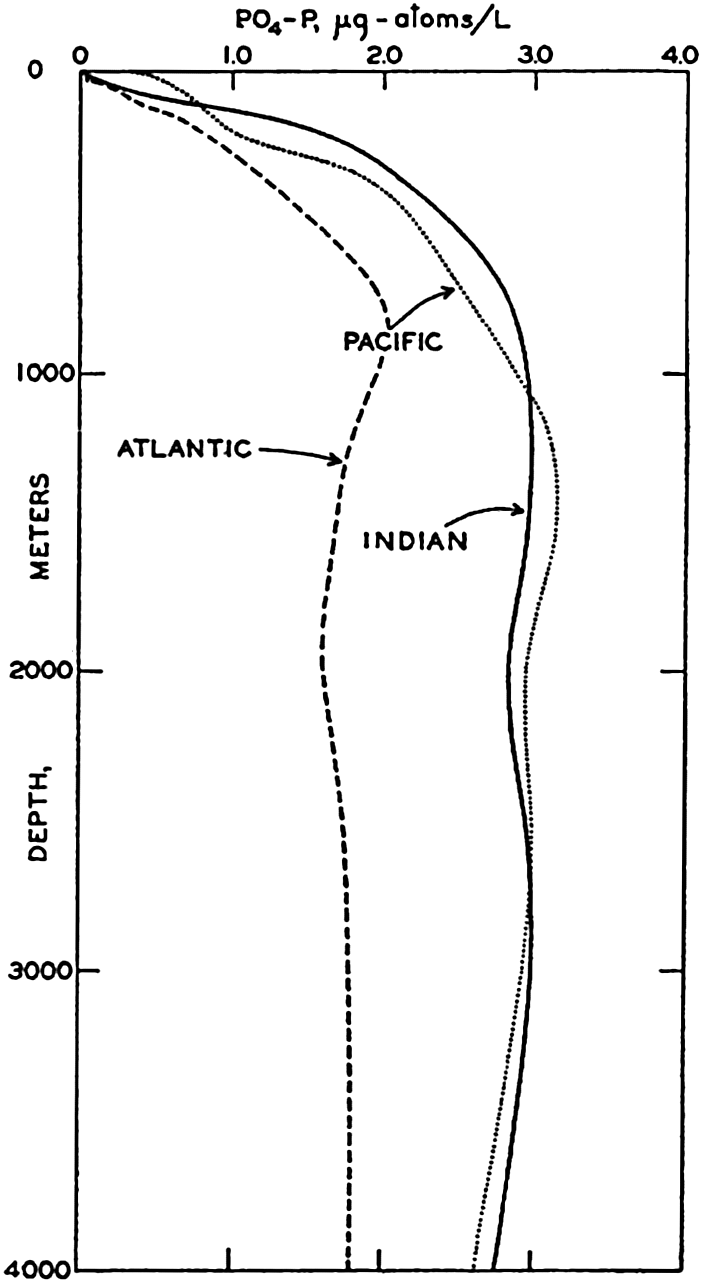
Check out this graph of phosphate vs depth in the ocean. It shows that looking at deep ocean values as a target doesn't match the surface:
http://publishing.cdlib.org/ucpress...r&chunk.id=d3_3_ch07&toc.id=ch07&brand=eschol
Vertical distribution of phosphate in the Atlantic, Pacific, and Indian Oceans based on data from the stations shown in fig. 44.
http://publishing.cdlib.org/ucpress...r&chunk.id=d3_3_ch07&toc.id=ch07&brand=eschol
Vertical distribution of phosphate in the Atlantic, Pacific, and Indian Oceans based on data from the stations shown in fig. 44.


















