- Joined
- Jan 5, 2019
- Messages
- 1,572
- Reaction score
- 902
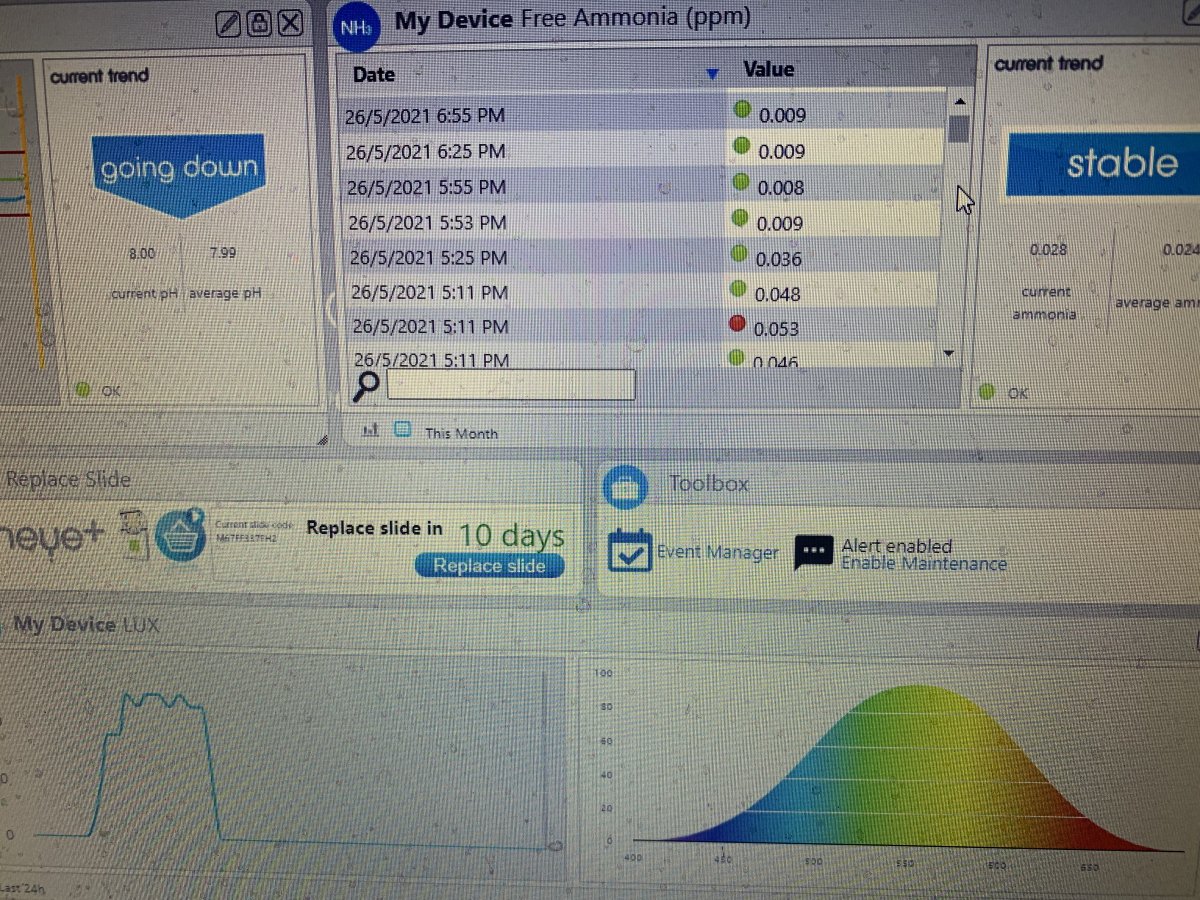
Well I can’t explain and I don’t know if this makes sense but the more ammonium chloride I dose the lower my No3 gets. My No3 is now below 1ppm.
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
I think the algae in the refugium and probably the coral are consuming ammonium and nitrate at the same time. I think the nitrate is dropping because the ammonium is stimulating growth of algae and coral, and the growth is causing the nitrate to drop(I think the nitrifying bacteria are probably not consuming most of the ammonium).Well I can’t explain and I don’t know if this makes sense but the more ammonium chloride I dose the lower my No3 gets. My No3 is now below 1ppm.
I’m going to try a three day blackout and see what happens to No3. Maybe I’m over lighting the refugium?I think the algae in the refugium and probably the coral are consuming ammonium and nitrate at the same time. I think the nitrate is dropping because the ammonium is stimulating growth of algae and coral, and the growth is causing the nitrate to drop(I think the nitrifying bacteria are probably not consuming most of the ammonium).
I think a three day refugium blackout sounds kind of excessive, IMO(I think it may create some big changes very quickly, a big increase in ammonia and/or nitrate). I may be ok, I'm not sure. You could dose nitrate(you could mix it with ammonium chloride), or you could do a combination of dosing nitrate and removing some algae from the refugium.I’m going to try a three day blackout and see what happens to No3. Maybe I’m over lighting the refugium?
Refugium light has been off for about 24 hours now and No3 is 1.5. I’ll turn it back on soon.I think a three day refugium blackout sounds kind of excessive, IMO(I think it may create some big changes very quickly, a big increase in ammonia and/or nitrate). I may be ok, I'm not sure. You could dose nitrate(you could mix it with ammonium chloride), or you could do a combination of dosing nitrate and removing some algae from the refugium.
That's not a very big increase in nitrate. Turning the light off for 24hrs may have been ok. I believe that dosing nitrate would be a better long term solution(I believe there would be more stability).Refugium light has been off for about 24 hours now and No3 is 1.5. I’ll turn it back on soon.
So, after an interesting discussion on nitrate dosing and looking at my Walt Disney coral and seeing it slightly less colorful than I have seen it in others tanks, I have decided I want to learn about and then try dosing ammonia instead of buying 20 damsels. Nitrate additives I see abound and have directions, but I see no ammonia additives for sale. In my limited knowledge, I understand stump remover is a form of ammonia, but I would have zero idea on how to make use of such . . .
so . . .
How would one get hold of ammonia? What calculations would be required? What would one monitor to check the impact?
And, no, I'm not going to run out and start dumping ammonia in my tank so don't worry. I'm just starting the learning process so I can understand what would be involved.
Thanks to any and all that have answers.
I'm very curious about the results. I wonder what the refugium light being off did to ammonia, nitrate, and alkalinity.Refugium light has been off for about 24 hours now and No3 is 1.5. I’ll turn it back on soon.
I only had the refugium light off for 24 hours. No increase in ammonia. I dosed 1ppm NeoNitro now No3 is 3.5ppm, alkalinity has been stable.I'm very curious about the results. I wonder what the refugium light being off did to ammonia, nitrate, and alkalinity.
If you reduce PAR in the refugium the algae probably would consume less nitrate, but then, the refugium probably wouldn't be as helpful with ph. There are probably at least a couple ways you can control the nitrate level in this situation, I think that dosing nitrate is probably the best way to do it if you want the fastest coral growth.I’m going to try a three day blackout and see what happens to No3. Maybe I’m over lighting the refugium?
This isn’t the first time No3 has been below 1ppm. I was running lights 24/7 on macros then No3 dropped below 1ppm I left the lights on 24/7 but dropped intensity, macros turned mushy and pale. Macros are looking much better now and I’m thinking less hours might be better? With how many fish I have and I feed them daily, I don’t think I should have to dose No3 just need to tune the refugium. Maybe I have too much macro algae and I should harvest more with leaving lights on 24/7?If you reduce par in the refugium the algae probably would consume less nitrate, but then the refugium probably wouldn't be as helpful with ph. There are probably at least a couple ways you can control the nitrate level in this situation, I think that dosing nitrate is probably be the best way to do it you want the fastest coral growth.
I didn't know you had the refugium light on and the reef tank light on at the same time. I think you should reduce the photoperiod of the refugium, starting at the end of photoperiod of the reef tank, and start shortening the photoperiod, going in reverse time. I think you should just experiment with it and see what works best.This isn’t the first time No3 has been below 1ppm. I was running lights 24/7 on macros then No3 dropped below 1ppm I left the lights on 24/7 but dropped intensity, macros turned mushy and pale. Macros are looking much better now and I’m thinking less hours might be better? With how many fish I have and I feed them daily, I don’t think I should have to dose No3 just need to tune the refugium. Maybe I have too much macro algae and I should harvest more with leaving lights on 24/7?
Heavy sigh . . . try reading before posting.Ammonium (NOT ammonia, which is not good for corals and just endangers everything in the tank; I can send you the study if you are interested) may help zooxanthellae in a nitrogen poor environment but honestly free form amino acids are the easiest way to provide nitrogen to corals. Dosing plain ammonia is unnecessarily dangerous and anyone who recommends it really needs to understand that there are much safer forms of nitrogen to give to a tank. A tank may be nitrogen starved, but ammonia starved is just a misunderstanding of what corals and such are actually using (the nitrogen part).

Ammonium (NOT ammonia, which is not good for corals and just endangers everything in the tank; I can send you the study if you are interested)
I wonder if this is related to the phenomena people experience with Seachem Prime. Prime converts ammonia into ammonium to detoxify, but Prime is known to reduce NO2 and NO3 levels, although Prime states the mechanism of exactly how is unknown. Following along!Well I can’t explain and I don’t know if this makes sense but the more ammonium chloride I dose the lower my No3 gets. My No3 is now below 1ppm.

I wonder if this is related to the phenomena people experience with Seachem Prime. Prime converts ammonia into ammonium to detoxify,
I'm not sure what you are getting at there, but it sounds wrong.
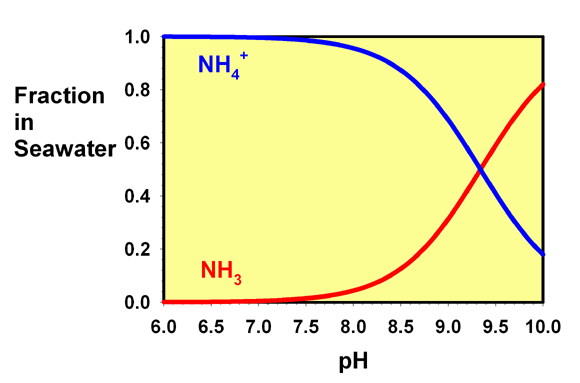
Regardless of whether you add ammonia (NH3) or ammonium (NH4+) to the water, both are instantly (far faster than you can blink an eye) converted into a mix of the two that depends only on the final pH, not which form you actually added.
Interesting, I always just assumed it was somehow Keeping it from going into its free form... I guess it is, but by binding it, and not by some black magic... The sulfur smell wasn't the devil after allNo, that is not correct. It is not simply ammonium. The only way to do that is by substantially lowering the pH (see graph below).
Treatments for Elevated Ammonia: Hydrosulfite and Bisulfite
A second type of compound used in commercial products (such as Seachem Prime) that claim to bind ammonia in marine aquaria is said to contain hydrosulfite (could be either HSO2- or - O2S-SO2-) and bisulfite (HSO3-). These compounds are well known dechlorinating agents, reducing Cl2 to chloride (Cl-), which process is also claimed to occur in these products. It is not apparent to me whether these ingredients actually react with ammonia in some fashion, or whether unstated ingredients in these products perform that function. Seachem chooses to keep the ingredients of their product secret, so aquarists cannot determine for themselves what is taking place, and how suitable it might be. Nevertheless, many aquarists seem to have successfully used products such as these to reduce ammonia's toxicity.
Note: products such as Seachem Prime hamper the ability to test for ammonia when using certain types of test kits (see above). Presumably, the product formed is still reactive with the Nessler reagents, even though it is not ammonia.
Figure 1. The fraction of free ammonia (NH3) and ammonium ion (NH4+) present in seawater as a function of pH.

Interesting, I always just assumed it was somehow Keeping it from going into its free form... I guess it is, but by binding it, and not by some black magic... The sulfur smell wasn't the devil after all

 www.fishforums.net
www.fishforums.net
