Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,142
- Reaction score
- 63,494
It pretty easy. It's by definition, grams / volume (mL) edit: only works for aqueous solutions.
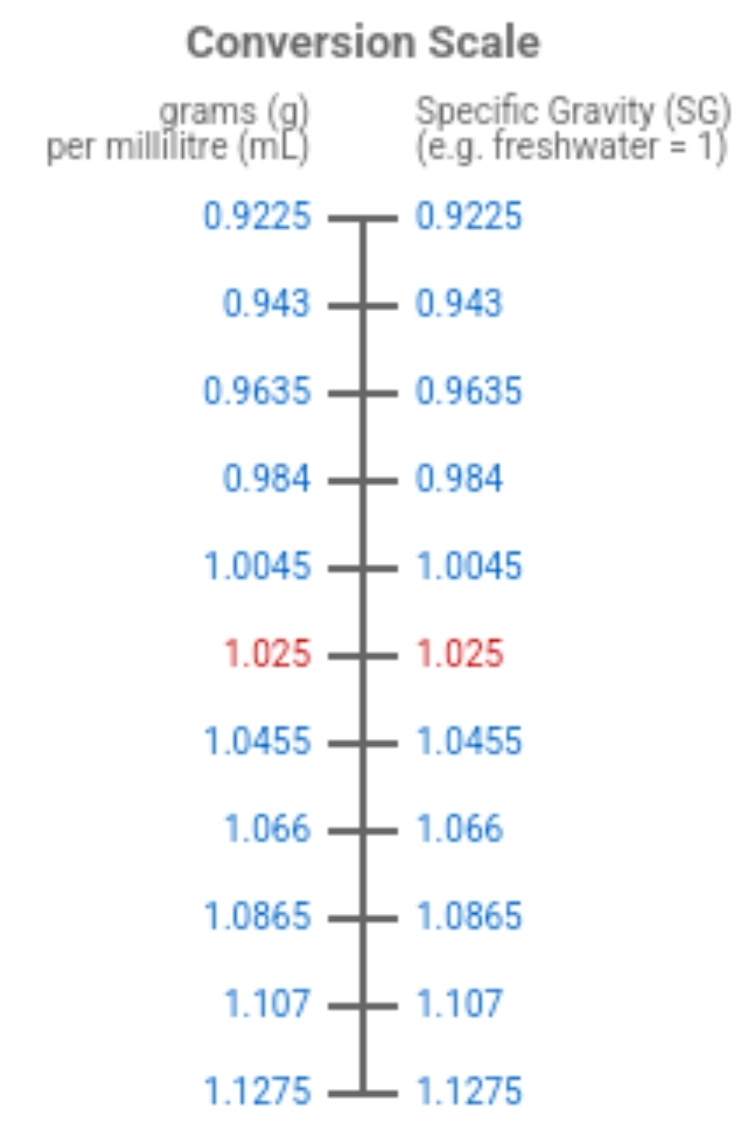
That oversimplifiction is why I asked. I know how to do it, and I was concerned you were doing it incorrectly based on your comments, and that appears to be the case.
One CANNOT assume freshwater is 1.000, because it is not except at 4 deg C.
To get from density to specific gravity, you have to look up the density of freshwater at the same temperature that you measured the density of your sample.
Then divide the density you determined by the density of fresh water at that temperature.



















