- Joined
- Aug 24, 2016
- Messages
- 1,504
- Reaction score
- 2,297
Super interesting article
Looks like a pretty large variation in both macro and trace elements. Also interesting that the zoox don't follow the same trend.
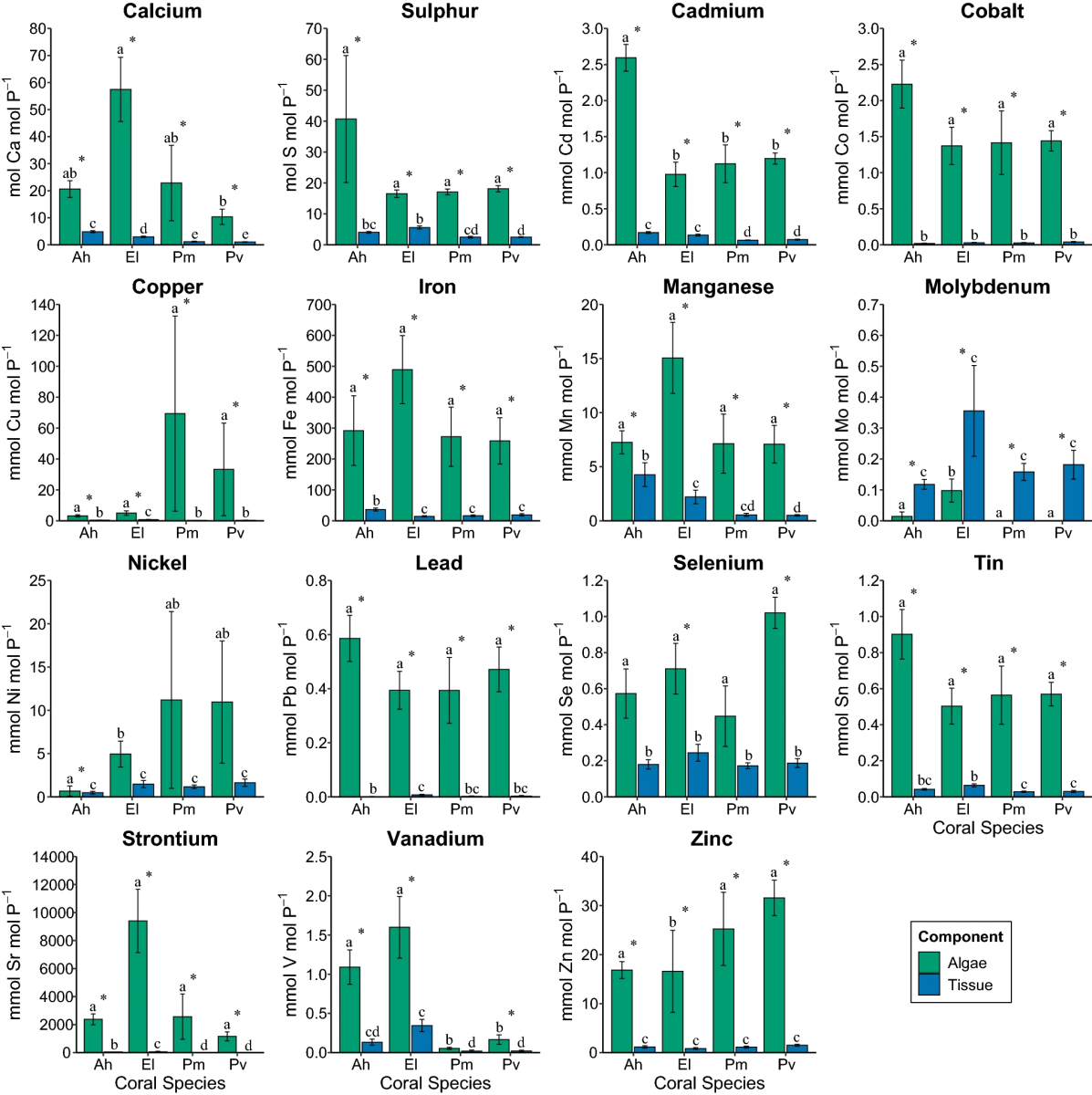
Indeed an interesting article. A similar investigation was already availabe which also analysed the skeletons. The only conclusion I can draw is that corals and zooxanthellae seem P-limited which is reflected by wide N : P ratios.
Unfortunately there is no connection between photophysiology and element ratios in the article. It would have been interesting if nutrient ratios influence photophysiology of individuals.
The practical use of the article is a bit limited because it is lacking skeletal analysis. Skeletons seem to reflect water concentrations better than tissue or symbionts. Nutrient uptake of symbionts and tissue is more regulated and possibly more influenced by feeding of the coral polyps.
Also if elements are just trapped in skeletons "by accident" they take up by far the highest proportion of metals and likely also of phosphate from the water, and it seems this process cannot be avoided.



















