- Joined
- May 22, 2016
- Messages
- 6,542
- Reaction score
- 10,099
Is growth in (some) reef tanks governed by trace element limitation (sometimes)?
Maybe? Probably?
The argument goes like this. If all macro nutrients are provided in abundance without care taken to replace the undetectable trace elements, then eventually some crucial scarce resource will be depleted to the point that growth slows. I should probably call these "micro-nutrients" instead of trace elements, because the limiting factor may not be an element per se, but perhaps some vitamin etc that is scarce and crucial.
Straightforward, but showing that this actually happens is a different thing.
I am keeping my tank replete in the macro nutrients: N, P, (in my case Si), Ca, Alk, Mg, and making sure my inputs of these nutrients stays absolutely consistent. Then measure anything informative I can measure to track growth in my system. In theory, if a micronutrient becomes limiting then the consumption of the macro nutrients should slow or stop, and their levels should rise with the same inputs.
Questions:
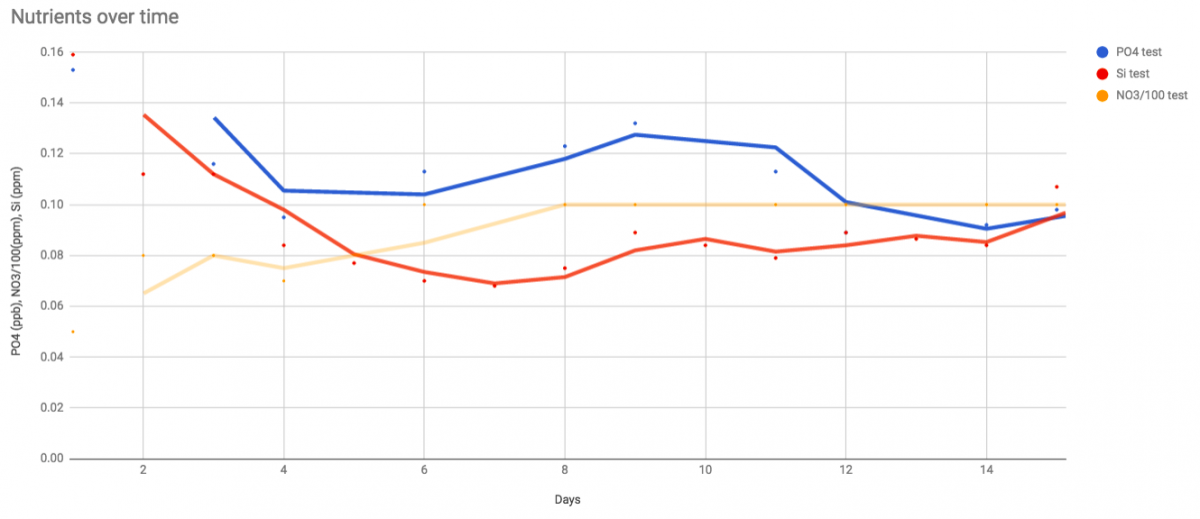
Line is a 2-day average of readings. NO3 I divided by 100 so it could all be on the graph. Not all 3 are precisely comparable. NO3 has huge error bars compared to the others, It's not necessarily steady at 10 - I just can't tell between 10 or 7 or 15ppm. Also it wasn't really moving opposite the other two the first few days, I just dosed more NO3 first few days to get it up to 10.
Si may look like consumption is slowing but it's not. Here's another graph showing same nutrients with the daily addition/consumption...
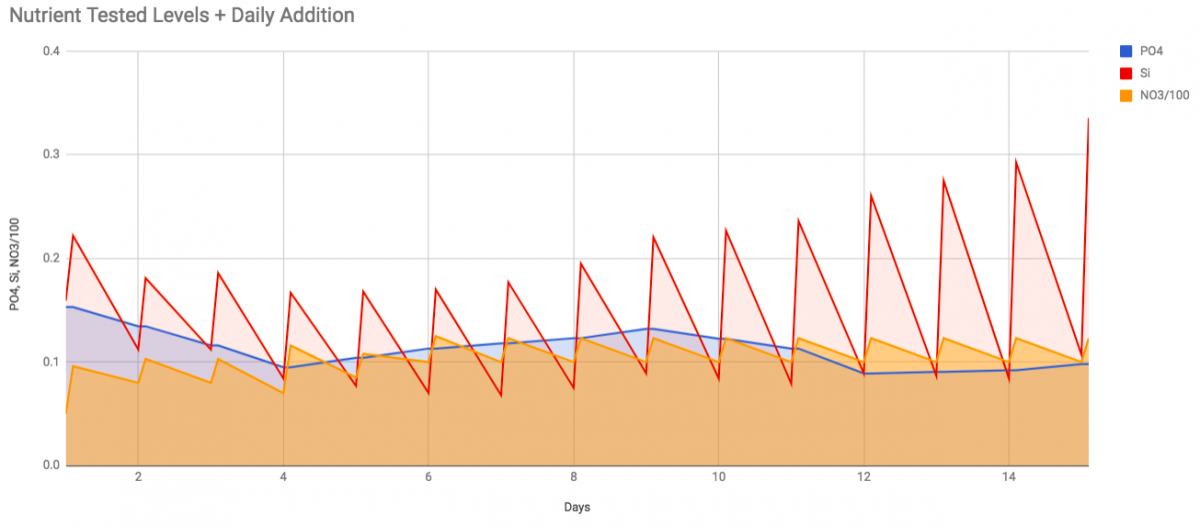
I'm upping Si dose 10% a day until I get to 1 ppm Si. I find it weird that Si consumption is increasing almost exactly at the same rate as the dose increase and drops back to near the exact same level every day. I'll redo this graph when I figure out how to calculate and show the food inputs of my 2 cubes a day to P & N.
One last graph - Ca, Alk, and pH. I had to scale the Ca weirdly to fit on the graph.
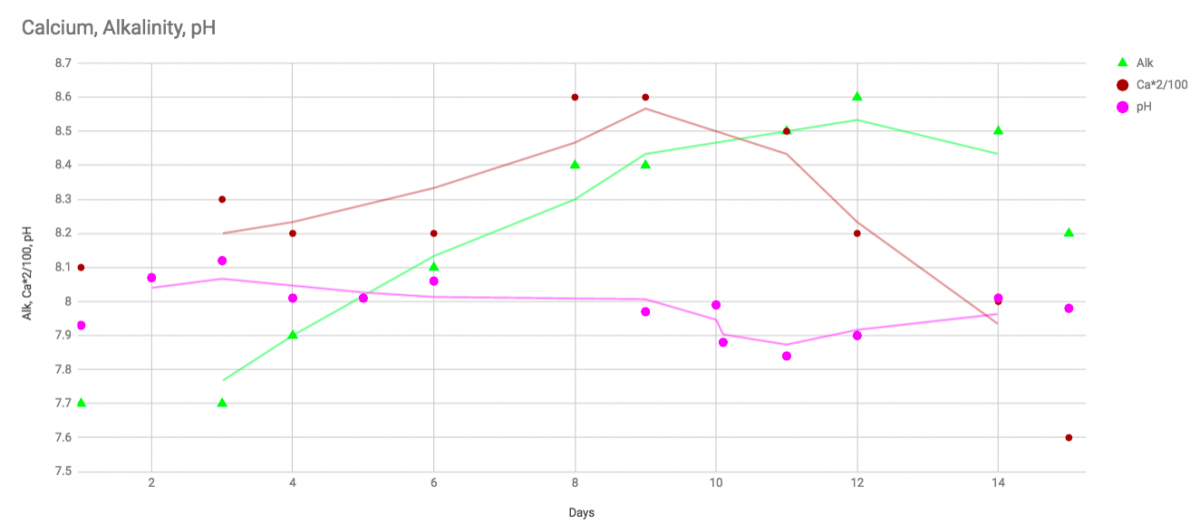
Final comment on the data for now - Most everything moved as expected for days 1-5. After adding a bunch of trace elements, the consumption generally increased. At first I thought the leveling out and the rise from day 6 to 9 was the onset of the limitation, but a likelier explanation is that days 7,8 & 9 we had cold cloudy weather, and my tank gets a few hours of natural morning sunlight from a window. Sun comes back out day 10 and following and growth pics back up.
Maybe? Probably?
The argument goes like this. If all macro nutrients are provided in abundance without care taken to replace the undetectable trace elements, then eventually some crucial scarce resource will be depleted to the point that growth slows. I should probably call these "micro-nutrients" instead of trace elements, because the limiting factor may not be an element per se, but perhaps some vitamin etc that is scarce and crucial.
Straightforward, but showing that this actually happens is a different thing.
I am keeping my tank replete in the macro nutrients: N, P, (in my case Si), Ca, Alk, Mg, and making sure my inputs of these nutrients stays absolutely consistent. Then measure anything informative I can measure to track growth in my system. In theory, if a micronutrient becomes limiting then the consumption of the macro nutrients should slow or stop, and their levels should rise with the same inputs.
Questions:
- Can growth be shown to slow dramatically in a tank with all major nutrients provided in abundance due to a depletion of micro nutrients?
- Can the limiting micro nutrient(s) be identified?
- Can the organism(s) being limited be identified?
Line is a 2-day average of readings. NO3 I divided by 100 so it could all be on the graph. Not all 3 are precisely comparable. NO3 has huge error bars compared to the others, It's not necessarily steady at 10 - I just can't tell between 10 or 7 or 15ppm. Also it wasn't really moving opposite the other two the first few days, I just dosed more NO3 first few days to get it up to 10.
Si may look like consumption is slowing but it's not. Here's another graph showing same nutrients with the daily addition/consumption...
I'm upping Si dose 10% a day until I get to 1 ppm Si. I find it weird that Si consumption is increasing almost exactly at the same rate as the dose increase and drops back to near the exact same level every day. I'll redo this graph when I figure out how to calculate and show the food inputs of my 2 cubes a day to P & N.
One last graph - Ca, Alk, and pH. I had to scale the Ca weirdly to fit on the graph.
Final comment on the data for now - Most everything moved as expected for days 1-5. After adding a bunch of trace elements, the consumption generally increased. At first I thought the leveling out and the rise from day 6 to 9 was the onset of the limitation, but a likelier explanation is that days 7,8 & 9 we had cold cloudy weather, and my tank gets a few hours of natural morning sunlight from a window. Sun comes back out day 10 and following and growth pics back up.




















