Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,421
- Reaction score
- 63,783
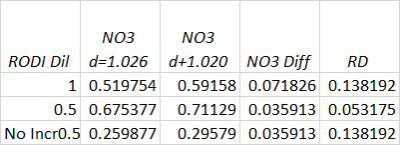
Yes just freshly made saltwater lol
SaltwaterQuairum said that Hanna told them (he said she said?) that the range can be extended to 0-50ppm with dilution
With the exact chloride level, I presume. I think it would be nice to see how sensitive it is to the natural variations folks see in both salinity and salt mix.