Hey it took me a year of reading everything I could and a year of actual trial and error to figure it all out I don't talk so good but I'm real good in math and most of whats happening in our tanks is a math problem a plus b equals Holly crap thats what it did experiences
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Help me understand low phosphates regarding Cyano Bacteria
- Thread starter Snoopdog
- Start date
- Tagged users None
You will get the hang of it just keep trying
Also the artical reference above pretty much awnsered my question thank you so much for sharing redfeild ratio
I´m not wrong and your link show thatSorry you are incorrect
In anaerobic respiration, a molecule other than oxygen is used as the terminal electron acceptor in the electron transport chain
Both inorganic and organic compounds may be used as electron acceptors in anaerobic respiration. Inorganic compounds include sulfate (SO42-), nitrate (NO3–), and ferric iron (Fe3+). Organic compounds include DMSO.
The bacateria in a deep sand bed use no3 and exhale so4
It is H2S (hydrogen sulphide) that is produced from existing sulphur compounds as a result of using sulphur compounds (SO4) as terminal electron acceptors. The bacteria responsible for this is not the same as the ones that are able to swing between oxygen and nitrate as electron acceptors depending on aerobic or anaerobic environment. Your link describe this very well
You use the word "sulfur" - if you instead use the word "hydrogen sulphide" you would be more right. Sulfur (or sulphur used on this side of the Atlantic) stands for an element. Elements can´t just appear or disappear in thin air. H2S is a compound and compound can change during time and different processes - both biological and non-biological.Many different types of electron acceptors may be used for anaerobic respiration. Denitrification is the utilization of nitrate (NO3−) as the terminal electron acceptor. Nitrate, like oxygen, has a high reduction potential. This process is widespread, and used by many members of Proteobacteria. Many denitrifying bacteria can also use ferric iron (Fe3+) and different organic electron acceptors.
Sulfate reduction uses sulfate (SO2−4) as the electron acceptor, producing hydrogen sulfide (H2S) as a metabolic end product. Sulfate reduction is a relatively energetically poor process, and is used by many Gram negative bacteria found within the δ-Proteobacteria. It is also used in Gram-positive organisms related to Desulfotomaculum or the archaeon Archaeoglobus.
Sulphur is not toxic as an element, neither as many compounds like sulphate - in fact your water contains around 900 ppm Sulphur (or sulfur in american). Please see the graph below - it show the content of Sulphur (S) in my aquarium for the last 4 years. On the other hand Hydrogen Sulphide (H2S) is very toxic but that is a compound between sulphur and hydrogen. If my sulphur in the graph has been in the form of H2S - everything - including myself have been dead. However - neither I or my aquarium is dead and haven´t been that the last 4 years
In fact - nitrate in the water suppress the bacteria responsible for the H2S production and that process just start when the denitrification is completed - H2S production is not as a byproduct of denitrification - it is its own process that start after the nitrate is "consumed"
Your link is excellent and describe the basics - the only remark I have is that even the denitrification need organic carbon compounds as electron donors, hence the use of these in reactors especially designed for denitrification.
I´m no expert in this but I have been working with water and water treatment for around 40 years and think that I have a rather good understanding of the processesses involved in anaerobic breakdown
Sincerely Lasse
Last edited:
Do you think this is valid for system like mine that´s run without scheduled WC? It have run the last 2.5 years without WC and my graph above indicate no tendency to accumulate during that time ant that you're right. The peak in early 2018 comes after a huge WC. However - I do not use any dry food in this aquaria - only frozen natural food. Could it be different in aquarium using dry food?Levels of sulfate vary widely between salt mixes and are far too small in foods to impact the levels, even ignoring that much of it ends up in organism tissues anyway.
Sincerely Lasse
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,304
- Reaction score
- 63,653
Do you think this is valid for system like mine that´s run without scheduled WC? It have run the last 2.5 years without WC and my graph above indicate no tendency to accumulate during that time ant that you're right. The peak in early 2018 comes after a huge WC. However - I do not use any dry food in this aquaria - only frozen natural food. Could it be different in aquarium using dry food?
Sincerely Lasse
I think we do not really know whether sulfate rises or falls over time (or both) in reef tanks without water changes. Both processes may happen, and like phosphate and nitrate accumulating or declining, its changes may depend on the fine details of the abiotic and biological processes as well as the foods and other additives.
- Joined
- Sep 21, 2018
- Messages
- 6,674
- Reaction score
- 7,169
I am not hidingHere it comes but this is ecology and it is not easy to test as a cause - effect in a petri dish. But can you repeat tests with the common used Guillard's F/2 nutrient media solution and if that not work as an mat forming agent - just add amino acids. I would first test with a solution like F/2 but without the vitamins. Next step - if nothing happens - ad the vitamins - and still no mat forming - ad some amino acids. Formula for F/2 - here You can by it too - seek for F/2 media
IMO fIf you can show that amino acids are essential for nitrogen uptake among mat building cyanobacteria - it would be a breakthrough. In my thought below I have assumed that
Your findings - if you are able to repeat them - corresponds with reports saying that adding amino acids sometimes can trigg cyanobacteria outbreak - but there is also outbreak in aquariums without adding amino acids - in fact it is very common in newly started aquariums without any internal production of amino acids. However - amino acids is a commonly used additive for low nutrient systems and probably common in poorly skimmed systems. Amino acids is the building blocks for proteins. Amino acids is an interesting organic nitrogen compound because it have been shown that the uptake of it in single cells are faster than the uptake of NH3/NH4. Cyanobacteria are either unicellular organisms or consist of a single cell layer with the cells in a row. Most micro algae is alike in cell construction including dinoflagellates. Your findings indicate that these benthic cyanobacteria can use amino acids and inorganic PO4 in the water column. So can microalgae too and if amino acids are an essential nitrogen source for cyanobacteria (you tests indicate that) it will not overturn my theory - instead it strengthens the theory in a complex ecosystem. However - I have to go back one or two steps and reinstall competition of space as an factor
In a ecosystem - benthic organisms compete for food and space and if different organism have the same demands - the speed of growth will decide which organism that will dominate. If food is there - the fight for space is the most important issue, hence growth speed have an enormous importance
For me - it's clear that normal microalgae have a faster growth than mat building cyanobacteria when all demands are satisfied. If we look at the two main combatants - cyanobacteria and microalgae I think that we can look at this first
Enhanced theory for triggers that induce mat forming if amino acids is cyanobacterias prefered nitrogen source.
- Both can use free inorganic P (as PO4)
- Cyanobacteria have a possibility to use PO4 produced by anaerobic bacteria below the mats either from metal bounded PO4 (with help of H2S) or through bacterial mineralization of organic matter. Microalgae in general can´t that with exception of dinoflagellates that can "dive" into the bottom substrate and pick up produced PO4. Maybe some other mobile microalgae can this too.
- Your experiments indicate that mat forming benthic cyanobacteria prefer amino acids (or maybe demand) as nitrogen source.
- Microalgae can use amino acids but also NH3/NH4 and for many of them NO3 too.
- In a given situation all of these nitrogen sources is evenly spread and will be used on an equal basis of each type of organisms - the amount of individuals of the different organisms will decide the use of it and how much space that it is occupied of each organism involved.
This is only free fantasies - it could be this way, it could be partly this way or it can total opposite to this way - but I hope this will stimulate the discussion
PO4 > 0.03 mg/l; all nitrogen sources constant above 0.4 mg/L as N
No change in competition
PO4 -> 0.03; Inorganic N sources constant; concentration of amino acid rise
As long as it is not to much rise of amino acids - no change in competition
PO4 -> 0; all nitrogen sources constant
Microalgae will have difficulties with growth - grow rate decline - their use of amino acids as nitrogen source decline. Result - more space and amino acids for the cyanobacteria will result in mat forming and suddenly they have free access to both PO4 and amino acids. PO4 rich environment will be occupied first (areas with organic matter and other stored PO4 sources - including newly dead coral tissue)
PO4 ->0; Inorganic N sources constant; concentration of amino acid rise
Speeding up the above process
PO4 steady above 0.03 mg/L Amino acid constant - inorganic nitrogen sources ->0
Microalgae will have difficulties with growth - grow rate decline - their use of amino acids as nitrogen source decline. Result - more space and amino acids for the cyanobacteria will result in mat forming and suddenly they have free access to both PO4 and amino acids. Amino rich environment will be occupied first (areas with organic matter - including newly dead coral tissue)
PO4 steady above 0.03 mg/L Amino acid rising - inorganic nitrogen sources ->0
Speeding up the above process
PO4 -> 0; amino acids constant or rising; Inorganic Nitrogen -> 0
Catastrophe
This is a try to explain the start of the problem. IMO – keeping a PO4 concentration above 0.03 – 0.05 mg/L, keeping inorganic nitrogen NH3/NH4 and/or NO3 above certain levels (NO3) or keep a flux of it (NH3/NH4) through the system and as low levels of amino acids as possible will prevent most cyanobacteria outbreaks in a large scale. However small outbreak on dead coral tissues or other organic concentrated waste can always happens.
How to act when an outbreak has happened.
Local outbreak
I normally just take a toothbrush and brush away the cyanobacteria from dead corals or just brush away organic matter. Check my nutrient levels
Heavy outbreak
Because of heavy outbreak normally spreads all over – it is – IMO – important too add NO3 (hinder H2S formation below the mats) and disturb the mats as much as possible. PO4 concentration above 0.05 and NO3 between 5-10 mg/L. If possible – lower the light intensity and let it slowly rise up too normal levels during 2 – 3 weeks. Stop all amino acid additions.
This trick have worked for me for many years and I know a lot of people that handle the problem the same way
Sincerely Lasse
I´m not wrong and your link show that
It is H2S (hydrogen sulphide) that is produced from existing sulphur compounds as a result of using sulphur compounds (SO4) as terminal electron acceptors. The bacteria responsible for this is not the same as the ones that are able to swing between oxygen and nitrate as electron acceptors depending on aerobic or anaerobic environment. Your link describe this very well
You use the word "sulfur" - if you instead use the word "hydrogen sulphide" you would be more right. Sulfur (or sulphur used on this side of the Atlantic) stands for an element. Elements can´t just appear or disappear in thin air. H2S is a compound and compound can change during time and different processes - both biological and non-biological.
Sulphur is not toxic as an element, neither as many compounds like sulphate - in fact your water contains around 900 ppm Sulphur (or sulfur in american). Please see the graph below - it show the content of Sulphur (S) in my aquarium for the last 4 years. On the other hand Hydrogen Sulphide (H2S) is very toxic but that is a compound between sulphur and hydrogen. If my sulphur in the graph has been in the form of H2S - everything - including myself have been dead. However - neither I or my aquarium is dead and haven´t been that the last 4 years
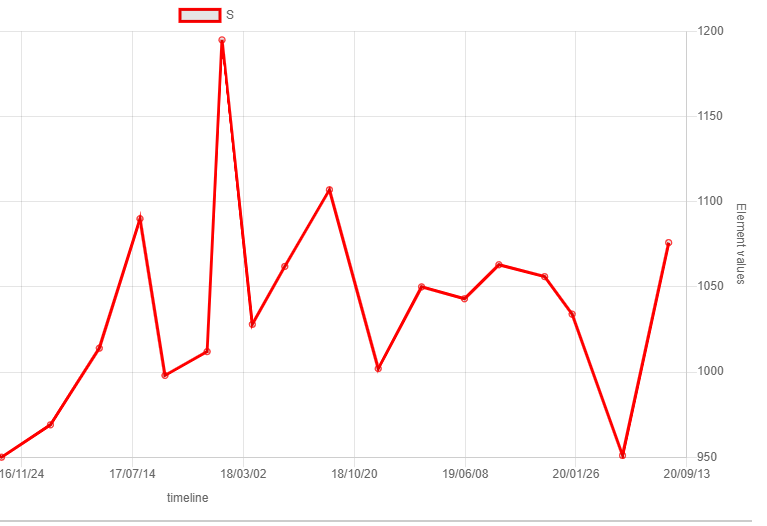
In fact - nitrate in the water suppress the bacteria responsible for the H2S production and that process just start when the denitrification is completed - H2S production is not as a byproduct of denitrification - it is its own process that start after the nitrate is "consumed"
Your link is excellent and describe the basics - the only remark I have is that even the denitrification need organic carbon compounds as electron donors, hence the use of these in reactors especially designed for denitrification.
I´m no expert in this but I have been working with water and water treatment for around 40 years and think that I have a rather good understanding of the processesses involved in anaerobic breakdown
Sincerely Lasse
I'll tell you what setup a deep sand bed let it run for 2 years then dig 4 to 6 inches down in the sand test the byproduct that is produced and then tell me its not sulphur as this is exactly what it is as I have done this myself while yes you are partially correct you are completely wrong about there not being s04 sulphur produced which then in turn has to be broken down by a compleatly diffrent layer benith that one and becouse that layer is not as effecient as the layer before it you end up over time with a s04 buildup that will eventually start leaching into the water the only way to avoid this is to have duel sand beds and every 2 years at 1 year and a half you start up the fresh bed and slowly transfer the bioload to the new bed over 6 months so you do not shock the system ending with the new bed taking over and the old bed being removed and replaced I have personal done this and seen the no3 consuming bacteria that expel s04 in action at a microbiogy lab as I have a good frjend who is a microbiologist and durring my learning curve I asked him alot of questions and he tought me alot showing me each and every thiing that was happening in my tank so I could achieve a mini ecosystem without crashing and killing everything I am speaking from first hand experience
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,304
- Reaction score
- 63,653
I'll tell you what setup a deep sand bed let it run for 2 years then dig 4 to 6 inches down in the sand test the byproduct that is produced and then tell me its not sulphur as this is exactly what it is as I have done this myself while yes you are partially correct you are completely wrong about there not being s04 sulphur produced which then in turn has to be broken down by a compleatly diffrent layer benith that one and becouse that layer is not as effecient as the layer before it you end up over time with a s04 buildup that will eventually start leaching into the water the only way to avoid this is to have duel sand beds and every 2 years at 1 year and a half you start up the fresh bed and slowly transfer the bioload to the new bed over 6 months so you do not shock the system ending with the new bed taking over and the old bed being removed and replaced I have personal does this and seen the no3 consuming bacteria that expel s04 in action at a microbiogy lab as I have a good frjend who is a microbiologist and durring my learning curve I asked him alot of questions and he tought me alot showing me each and every thiing that was happening in my tank so I could achieve a mini ecosystem without crashing and killing everything I am speaking from first hand experience
FWIW, I had a deep fine oolitic aragonite bed for a couple of years. I'm not sure it did anything useful, but when I removed it, there was no evidence of hydrogen sulfide/metal sulfides at any depth.
And since we want to get technical the exact prosses is as folows 02,no3-,se04squared- and finally s04squared I skipped the nitrogen cycle prosses in this going from 02 to n03 as most of us if not all of us should know how this happens
Last edited:
Then you start the sulphur cycle which is an entirely different cycle starting with s04
Had to find the old journals I wrote while learing all this
I'm not saying that hydrogen sulfide is not also produced by an entirely different bacteria but what I am saying is s04 is produced and will build up and leach into the water if not taken care of manually
If it didnt do anything useful You may not of been feeding it as you can starve a sand bed just like any other organism I used my dsb as the sole means of nitrogen removal from the system with the exception of the red mangroves using some and leaves shedding that were removed but my setup produced an imbalance of phosphates and nitrogen so I ended up with more nitrogen in the system then could be used hense the need for a deep sand bed to remove the excess and why the deep sand bed never became a phosphate sink hole leading eventually to leaching phosphatesFWIW, I had a deep fine oolitic aragonite bed for a couple of years. I'm not sure it did anything useful, but when I removed it, there was no evidence of hydrogen sulfide/metal sulfides at any depth.
Also my deep sand bed was 24 inches deep not your standered 4 to 6
But I'm done debating this everyone has had valid points each experience is diffrent depending on what goes into the system and how the system itself is setup to run I have shown what is happening and need not defend myself anymore I will move on to someone else I can help the question was asked how can sulphur enter a system with high nitrogen and no water changes and I have answered that the best wishes to all
Last edited:
- Joined
- May 18, 2018
- Messages
- 432
- Reaction score
- 602
Never had did experience with my sandbeds including a 10 year old remote dsb.I'll tell you what setup a deep sand bed let it run for 2 years then dig 4 to 6 inches down in the sand test the byproduct that is produced and then tell me its not sulphur as this is exactly what it is as I have done this myself while yes you are partially correct you are completely wrong about there not being s04 sulphur produced which then in turn has to be broken down by a compleatly diffrent layer benith that one and becouse that layer is not as effecient as the layer before it you end up over time with a s04 buildup that will eventually start leaching into the water the only way to avoid this is to have duel sand beds and every 2 years at 1 year and a half you start up the fresh bed and slowly transfer the bioload to the new bed over 6 months so you do not shock the system ending with the new bed taking over and the old bed being removed and replaced I have personal done this and seen the no3 consuming bacteria that expel s04 in action at a microbiogy lab as I have a good frjend who is a microbiologist and durring my learning curve I asked him alot of questions and he tought me alot showing me each and every thiing that was happening in my tank so I could achieve a mini ecosystem without crashing and killing everything I am speaking from first hand experience
Yes now when you are proven wrong - you have in all your post said that sulphur is produced and that´s totally wrong. And it is also total wrong that SO4 will be produced in an anaerobic bed - where should the O come from? Neither S or SO4 is toxic or have the smell of rotten eggs. However SO4 is used (Sulfate - not elementary sulphur - in this case with heterotrophic bacteria) as an electron acceptor and the end product is H2S (hydrogen sulfide) and it is toxic and smell like rotten eggs.I'm not saying that hydrogen sulfide is not also produced by an entirely different bacteria but what I am saying is s04 is produced and will build up and leach into the water if not taken care of manually
My tank water hold around 1000 mg/L of sulphur (calculated as S) but in elementary sulphur does not exist as an ion in the water column. instead it exist as sulfate (SO4) and the content of sulfate in my water is around 3000 mg/L. That´s much and the concentration of sulfate in the capillary water of the sandbed will be the same. Hence there is enough of sulfate already present - it does not need to be a uppbuild. During anaerobic environment and in the absence of nitrate - this sulfate will be used and hydrogen sulphide will be formed. Hydrogen sulphide (H2S) is a gas and will leave the gravel for the water column and if it is oxygen in the water - will it be oxidized directly to sulfate again (if I am wrong - I am no chemist - @Randy Holmes-Farley, please correct me).
The way to control that no or minor hydrogen sulfide will be produced in a sandbed is to not let the NO3 level hit zero and the fail back is that it should be in contact with oxygen rich water in the interface between water and sand bed. However if a power shout of happens - the oxygen will be consumed fast and so also the nitrate. That´s the reason why I have my DSB in my refugium. A nitrate concentration of around 2 ppm is my control of H2S production. It is a type of remote DSB with fast flow and oxygen production during night time (reversed light over the refugium)
Yes do that so you get the nomenclature right and name apples for apples and not bananasHad to find the old journals I wrote while learing all this
No - the science is the same in every tank - it is only the aquarists explanations that differBut I'm done debating this everyone has had valid points each experience is diffrent depending on what goes into the system and how the system itself is setup to run I have shown what is happening and need not defend myself anymore I will move on to someone else I can help the question was asked how can sulphur enter a system with high nitrogen and no water changes and I have answered that the best wishes to all
Yes me tooFWIW, I had a deep fine oolitic aragonite bed for a couple of years. I'm not sure it did anything useful, but when I removed it, there was no evidence of hydrogen sulfide/metal sulfides at any depth.
Sincerely Lasse
o is carried down threw the entire prosses as anaerobic means a lack of dissolved oxygen not bound oxygen and as n is used s is expelled see post
and at this point yes s04 starts its cycle ending in h2s. But as stated this prosses is less effecient then the rest and ends up with a build up of so4 as n03 is used and converted much faster but really I'm done this time as it seems like no matter what I show you will always have to be right also you did not prove me wrong in anyway at all you told me that I dont have a stone from Harry Potter to convert n to s and I was showing you that it is done threw micro organisms you also asked how sulphur can get into a tank with alot of nitrogen and no water changes I awnsered that I hope one day you get to see exactly what I'm talking about unfortunately if you do it'll be the end of your current tank as an over abundance of anything can and will killAnd since we want to get technical the exact prosses is as folows 02,no3-,se04squared- and finally s04squared I skipped the nitrogen cycle prosses in this going from 02 to n03 as most of us if not all of us should know how this happens
Last edited:
Good luck and best wishes
Similar threads
- Replies
- 11
- Views
- 334
- Replies
- 11
- Views
- 339
- Replies
- 9
- Views
- 192
- Replies
- 7
- Views
- 124
- Replies
- 1
- Views
- 89
New Posts
-
-
Nebraska Live Goods SPONSOR Phyto and Pods! ALL YOUR REEF CAN EAT
- Latest: Reef By Steele


















