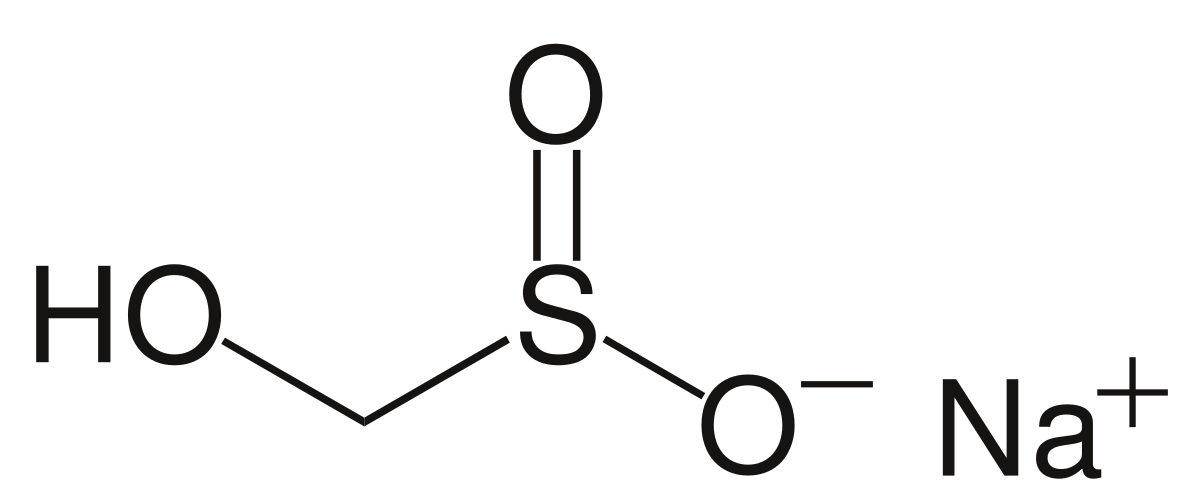
Hi @Randy Holmes-Farley
Out of curiosity, do you know how exactly does Prime 'detoxify' ammonia and nitrite? There are no answers from Google, and this has just been something I have been so curious about.
Out of curiosity, do you know how exactly does Prime 'detoxify' ammonia and nitrite? There are no answers from Google, and this has just been something I have been so curious about.