Thanks great write up
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
How Much Two Part Do I Need?
- Thread starter redfishbluefish
- Start date
- Tagged users None
Seeing the great diagram and formula around coral calcification - does dosing lots of two part raise your salinity meaningfully if it's producing NaCl as an output?
I would imagine it does not.
I would imagine it does not.
Awesome job! Ok once I get my parameters where I wanted to be at should I start dosing the 2 part equally or dose what I need to maintain it?
In the ideal world (stoichiometry of the two compounds), you would dose the same amount of alk as calc. However, I've found that my dosing is slightly off, maybe because of the salt I'm using, or that I never calibrated my 1.1 ml/min dosing pumps, and simply go by time (the pumps differ by a couple minutes some times), or that the solutions aren't perfectly made to the correct concentration (I use measuring cups, when weight would be more accurate). So once you get your parameters where you want them, I'd start with the same volumes of alkalinity and calcium, and make fine adjustments once you see what's going on.
Seeing the great diagram and formula around coral calcification - does dosing lots of two part raise your salinity meaningfully if it's producing NaCl as an output?
I would imagine it does not.
The simple answer is "Yes" it raises salt.....Randy talks about this in the DIY article linked in the OP (and maybe other articles, but I know he talked about it there).
However, in practice, and I've been doing this since 2007, I've never noticed an increase or adjusted for it. I'm guessing that water changes and the saltwater lost to the skimmer (and replaced with RO/DI water) keep my salt concentration steady.
Awesome job! Ok once I get my parameters where I wanted to be at should I start dosing the 2 part equally or dose what I need to maintain it?
You should always dose what you need. All tanks are different. All products are different and therefor dose differently.
My tank for instance is using 260ml of alk per day and I think 147ml of calcium and right at 140 ml of magnesium. Some tanks never add magnesium but as you can see with me adding so much I really need to. I go for the numbers I want to maintain for each element and dose according to that. My targets are very near full strength natural seawater.
Trial and error or test and adjust is the only way you can figure out what your tank needs. No label or internet recommendation can tell you what to dose.
Do you mind sharing your parameters? Also is there a balance scale between Alk and Cal or is it whatever I want it to be? I.e. The balance of 9dKH Alkalinity is 424ppm Calcium, 10.0dKH Alkalinity is 431ppm Calcium and so on? I'm new to dosing so I'm still trying to get a better understanding. Thanks!You should always dose what you need. All tanks are different. All products are different and therefor dose differently.
My tank for instance is using 260ml of alk per day and I think 147ml of calcium and right at 140 ml of magnesium. Some tanks never add magnesium but as you can see with me adding so much I really need to. I go for the numbers I want to maintain for each element and dose according to that. My targets are very near full strength natural seawater.
Trial and error or test and adjust is the only way you can figure out what your tank needs. No label or internet recommendation can tell you what to dose.
- Joined
- Oct 12, 2014
- Messages
- 15
- Reaction score
- 11
Yes, this is my issue as well...my calcium is usually where I want it but I want to boost by alk about 1dkh...if I dose both will my calcium continue to rise as well? I currently dose kalk, which has been keeping my system stable but I want to increase alk, as I am trying to prompt better growth.
Yes, the calculation is correct for 50 liters of total volume and using SODA ASH solution (baked baking soda). However, as mentioned, you don't want to raise alkalinity that fast. Do it slowly over 2 to 3 days.
I also want to clarify BAKING SODA solutions versus SODA ASH. In your first post above, you mention baking soda (un-baked). Note the solubility of baking soda is roughly half that of soda ash. If you make up a solution of baking soda to use as an alk supplement, it's half the strength of the soda ash solutions, and therefore would require twice that volume to get the same increase in dKH (47.1mls). Hope I made myself clear on this.
Thanks Paul for your answer, actually what I meant is that my daily dkh consumption is of 2.5dkh
- Joined
- Jan 23, 2016
- Messages
- 1,707
- Reaction score
- 1,614
Great read Paul. And I read it twice. I just started dosing BRS, and still figuring things out. I'm 1 1/2 years in. Still don't have a big need, but WC wasn't enough. I've been fighting high No3's, and started Vinegar dosing, which increases concentrations.
Et817 there is a balance chart on the Reefgrow website. I believe at the bottom of the page. Keep in mind that our alkalinity kits read low (I think). At least many do. They claim to read total alkalinity but I haven't seen that yet.
I try to keep my Alk between 7 and 8 dkh using my Hanna checker or Salifert kit even though I know this is a low reading. My best guess is I am truly between 8 and 9. My tank is fairly low nutrient and I have issues when I try to get to nsw levels.
Calcium I keep between 380 and 425. Magnesium anywhere between 1250 and 1350.
My dosing is getting pretty crazy lately where I am upping my amounts 5 to 10 ml a week so it is hard to keep really stable and I test 4 to 7 times a week.
I always test around the same time in the evening. Give or take an hour.
I try to keep my Alk between 7 and 8 dkh using my Hanna checker or Salifert kit even though I know this is a low reading. My best guess is I am truly between 8 and 9. My tank is fairly low nutrient and I have issues when I try to get to nsw levels.
Calcium I keep between 380 and 425. Magnesium anywhere between 1250 and 1350.
My dosing is getting pretty crazy lately where I am upping my amounts 5 to 10 ml a week so it is hard to keep really stable and I test 4 to 7 times a week.
I always test around the same time in the evening. Give or take an hour.
- Joined
- Sep 25, 2016
- Messages
- 23
- Reaction score
- 12
How Much Two Part Do I Need?
I'm a two part doser for alkalinity and calcium (and magnesium), and have been repeatedly asked about how to figure out how much of each a new two-parter needs to dose to their tank. This subject has been thoroughly covered, so this article will predominantly be links to this information, and include my opinion and personal preferences.
What I'll review is:
A. Understanding Two Part Chemicals
B. The Need for Test Kits
C. A Video Summary on Two Part
D. So How Much Do I Need
A. Understanding Two Part Chemicals
The two parts are made up of an alkalinity part and a calcium part. The alkalinity part is commonly sodium bicarbonate (baking soda) or sodium carbonate (soda ash), while the calcium part is calcium chloride (associated with an amount of water). For alkalinity, I prefer soda ash for two reasons:
1. It's more soluble in water and therefore I can make a "strong" solution.
2. It pushes pH up oh so slightly more than baking soda.
The least expensive way that I've found to dose alkalinity is to make your own by simply purchasing baking soda and baking it in the oven for an hour. I purchase large bags from a club store for around 6 dollars for 13.5 pounds, which will make about 12 gallons of alkalinity solution. Simply spread 2 1/4 cups onto a baking sheet and bake at 350 for one hour. You have now converted baking soda to soda ash. Here's two batches ready to go into the oven:

Once they come out, mix with RO/DI water to make one gallon solutions. (With this example above, each cookie sheet has 2 1/4 cups of baking soda, enough to make 2 gallons of alkalinity solution in total.) Also note that once baked, the volume has been reduced to about 2 cups of soda ash on each sheet. You now have alkalinity solution to dose to your tank, and it cost you pennies.
When choosing a calcium part (when you're doing it by DIY), you need to have a rough estimate of the amount of bound water. For those using Dow Flakes (if you can find them), or BRS calcium, you need 2 1/2 cups per gallon of water, because it has a fair amount of water associated with the calcium chloride. For those using a more anhydrous form (less bound water), of calcium (such as Prestone Driveway Heat), you only need two cups per gallon. So for DIY'ers, take note of the water content of your calcium and adjust accordingly....between 2 to 2 1/2 cups per gallon.
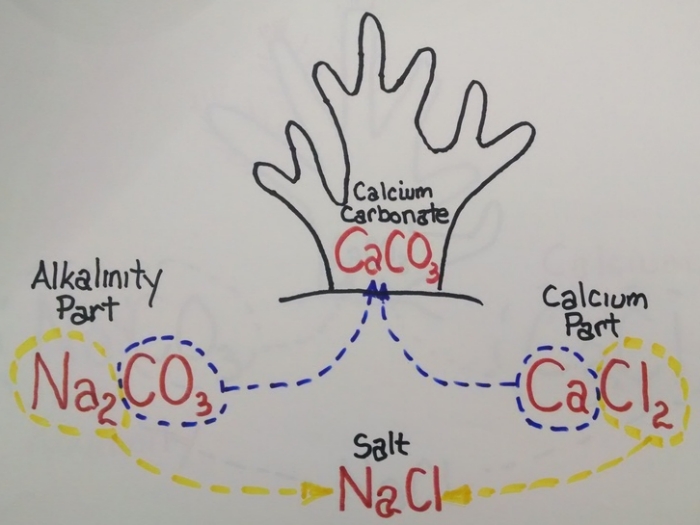
Now, why do hard corals (and clams) need two part? The simplified chemistry is quite elegant. The corals take the carbonate from the alk part and the calcium from the calc part and combine the two to make calcium carbonate (the hard part of corals and clams), with the sodium and chloride part combining to make salt.
Na2CO3 + CaCl2 ----> CaCO3 + NaCl
Alk Part + Calc Part -----> Calcium Carbonate + Salt
Our own Dr. Randy Holmes Farley has written a number of articles on this subject, and suggest reading them to fully understand what is occurring with these two additives in our tanks:
The “How To” Guide to Reef Aquarium Chemistry for Beginners, Part 2: What Chemicals Must be Supplemented
Calcium and Alkalinity
A Simplified Guide to the Relationship Between Calcium, Alkalinity, Magnesium and pH
If you wish to delve further into the biochemistry of corals consuming calcium carbonate, Randy covers that in this article:
The Chemical and Biochemical Mechanisms of Calcification
And finally, if you wish to DIY your own two part, including calcium and magnesium, that is covered in this one:
An Improved Do-it-Yourself Two-Part Calcium and Alkalinity Supplement System
BRS uses Randy's recipe in the chemicals they sell, with no baking of baking soda needed because they sell soda ash directly. FYI, I bake my baking soda at 350 F instead of 300 mentioned in the above article.....doesn't hurt anything, and assures all is converted to the carbonate form. Note that extra time in the oven isn't critical either, so anything over an hour is fine.
B. The Need for Test Kits
If you're going to dose two part (or for that fact, any other means of maintaining alkalinity and calcium), you need a way of testing alkalinity, calcium and magnesium. I know you hate to do it, but I'm going to repeat, You Need To Test! The common choices are either Hanna checkers or wet chemistry test kits (titrations). For those not comfortable with wet chemistry, Hanna checkers are the way to go. Two errors I commonly see with Hanna's are:
1. Not cleaning the outside of the cuvette (vial) before putting it in the tester...finger prints need to be gone!
2. Putting the vial in the tester with liquid on the outside (which could ruin your tester).
So wipe it clean before going into the tester. You don't want to be putting this smudged up, wet vial into your Hanna Checker:

With wet chemistry methods, I am very familiar with API and Red Sea kits, both of which use titration as a means to determine both alkalinity and calcium. I am not aware of any alkalinity or calcium test kit that requires matching the color of the solution to a particular color on a card, typical of API nitrate, nitrite and ammonia kits, just to mention a few. If such color matching kits exist for alkalinity and calcium, I personally would not like them because I have a very difficult time matching the color in the vial to the color on the card. For some reason, when using these color matching kits, the color I get in the vial doesn't match any of the colors on the card. I should point out that I am colorblind, and that might very well be the reason I have difficulty with these kits. With the titration alkalinity and calcium kits—where I'm just looking for a color change—I have no problem.
So API and Red Sea (and others) are simple color changing titration kits, and my preference with these is the Red Sea Pro kit. This Pro kit includes all three test kits in one package; alkalinity, calcium and magnesium kits. This kit simply changes colors when the titration is done.

The common errors I see folks making with these kits are:
1. Not following instructions. Even though I've done these tests hundreds of times, I still pull out the summary card and follow the instructions explicitly. This is especially true with the magnesium test (from Red Sea), where you need to add five drops of a solution...see that little x5 on the card.
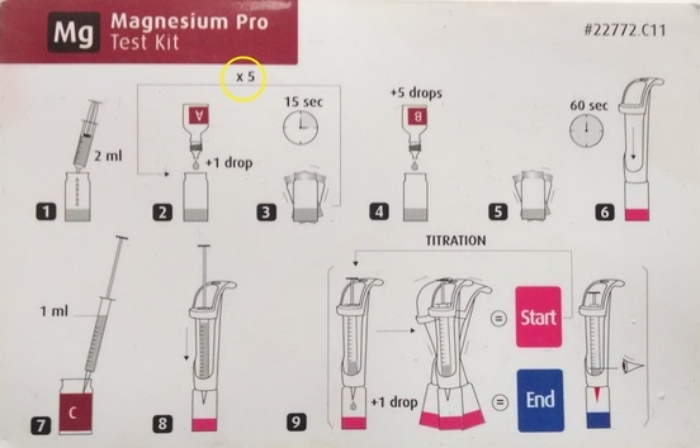
2. Thinking they need to match the color on the summary card to the color in the vial. The titration is over when it changes for one color to the other, where one more drop has no effect on the color. It's not like the test kits where you are matching the exact color on the card.
3. Reading the syringe wrong. With Red Sea, the syringe, when full is 1 ml. When you are done with the titration, the amount you put into the vial IS NOT THE READING ON THE SYRINGE. You need to take that reading on the syringe and subtract it from 1. So if the syringe says 0.4, the amount you used is 1 - 0.4 = 0.6 mls.
C. A Video Summary on Two Part
I'm including this video from BRS because I thought they did a good job of summarizing the two part system.
How To: Dosing Two Part in Your Reef Tank
D. So How Much Do I Need
Even though this has been covered in the linked articles and video above, I'm going to summarize my preferences in how to determine the amount of alkalinity and calcium I need to add to my tank on a daily basis.
INITIAL TESTING ----> ADJUST TO IDEAL LEVELS ---> DETERMINE CONSUMPTION ---> BEGIN DOSING
First thing is to test your alkalinity, calcium and magnesium levels and adjust them to where you would like them to be. Recommended levels are calcium around 400ppm, alkalinkity 7 - 11 dKH, and magnesium 1380 - 1400 ppm. When you do your original testing, use a reef calculator to determine how much you'll need to bring up your levels to where you'd wish them to be. I use the BRS calculator found HERE to figure out how much I'll need. Note that with calcium, you don't want to raise more than 50 ppm per day, and with alkalinity I like to raise no more than 1 dKH per day, until I'm where I want to be. When adding alkalinity and calcium, you want to add these in a high flow area, and not at the same time. If you add them at the same time, you'll get immediate precipitation of calcium carbonate. So separate the additions by a couple minutes to allow the solution to disperse.
Once you've gotten your numbers where you want them, test again about 24 hours later and note the drop. If you have an established tank with colonies of hard corals (and clams), you can use this number to determine your daily consumption, and again, using the calculator to determine how much you need to start dosing to maintain those levels. If you only have a few hard corals or just frags, you may wish to test again on day 2 and maybe day 3 to get a better idea of your daily consumption, and now use that number to determine dosing.
I would now recommend daily testing to make fine adjusts to the amounts you are dosing. Note with Magnesium, I do not dose daily. Dependent on the salt you are using, you may only need to dose weekly or monthly with magnesium. Continue daily testing and adjustments until you are confident in the way your tank is consuming alkalinity and calcium. Once stabilized, you can back off on testing to maybe weekly, or whatever time period you feel comfortable doing, knowing your tank numbers aren't moving.
Now sit back and watch your corals grow.
- Joined
- Sep 25, 2016
- Messages
- 23
- Reaction score
- 12
How Much Two Part Do I Need?
I'm a two part doser for alkalinity and calcium (and magnesium), and have been repeatedly asked about how to figure out how much of each a new two-parter needs to dose to their tank. This subject has been thoroughly covered, so this article will predominantly be links to this information, and include my opinion and personal preferences.
What I'll review is:
A. Understanding Two Part Chemicals
B. The Need for Test Kits
C. A Video Summary on Two Part
D. So How Much Do I Need
A. Understanding Two Part Chemicals
The two parts are made up of an alkalinity part and a calcium part. The alkalinity part is commonly sodium bicarbonate (baking soda) or sodium carbonate (soda ash), while the calcium part is calcium chloride (associated with an amount of water). For alkalinity, I prefer soda ash for two reasons:
1. It's more soluble in water and therefore I can make a "strong" solution.
2. It pushes pH up oh so slightly more than baking soda.
The least expensive way that I've found to dose alkalinity is to make your own by simply purchasing baking soda and baking it in the oven for an hour. I purchase large bags from a club store for around 6 dollars for 13.5 pounds, which will make about 12 gallons of alkalinity solution. Simply spread 2 1/4 cups onto a baking sheet and bake at 350 for one hour. You have now converted baking soda to soda ash. Here's two batches ready to go into the oven:

Once they come out, mix with RO/DI water to make one gallon solutions. (With this example above, each cookie sheet has 2 1/4 cups of baking soda, enough to make 2 gallons of alkalinity solution in total.) Also note that once baked, the volume has been reduced to about 2 cups of soda ash on each sheet. You now have alkalinity solution to dose to your tank, and it cost you pennies.
When choosing a calcium part (when you're doing it by DIY), you need to have a rough estimate of the amount of bound water. For those using Dow Flakes (if you can find them), or BRS calcium, you need 2 1/2 cups per gallon of water, because it has a fair amount of water associated with the calcium chloride. For those using a more anhydrous form (less bound water), of calcium (such as Prestone Driveway Heat), you only need two cups per gallon. So for DIY'ers, take note of the water content of your calcium and adjust accordingly....between 2 to 2 1/2 cups per gallon.
Now, why do hard corals (and clams) need two part? The simplified chemistry is quite elegant. The corals take the carbonate from the alk part and the calcium from the calc part and combine the two to make calcium carbonate (the hard part of corals and clams), with the sodium and chloride part combining to make salt.
Na2CO3 + CaCl2 ----> CaCO3 + NaCl
Alk Part + Calc Part -----> Calcium Carbonate + Salt

Our own Dr. Randy Holmes Farley has written a number of articles on this subject, and suggest reading them to fully understand what is occurring with these two additives in our tanks:
The “How To” Guide to Reef Aquarium Chemistry for Beginners, Part 2: What Chemicals Must be Supplemented
Calcium and Alkalinity
A Simplified Guide to the Relationship Between Calcium, Alkalinity, Magnesium and pH
If you wish to delve further into the biochemistry of corals consuming calcium carbonate, Randy covers that in this article:
The Chemical and Biochemical Mechanisms of Calcification
And finally, if you wish to DIY your own two part, including calcium and magnesium, that is covered in this one:
An Improved Do-it-Yourself Two-Part Calcium and Alkalinity Supplement System
BRS uses Randy's recipe in the chemicals they sell, with no baking of baking soda needed because they sell soda ash directly. FYI, I bake my baking soda at 350 F instead of 300 mentioned in the above article.....doesn't hurt anything, and assures all is converted to the carbonate form. Note that extra time in the oven isn't critical either, so anything over an hour is fine.
B. The Need for Test Kits
If you're going to dose two part (or for that fact, any other means of maintaining alkalinity and calcium), you need a way of testing alkalinity, calcium and magnesium. I know you hate to do it, but I'm going to repeat, You Need To Test! The common choices are either Hanna checkers or wet chemistry test kits (titrations). For those not comfortable with wet chemistry, Hanna checkers are the way to go. Two errors I commonly see with Hanna's are:
1. Not cleaning the outside of the cuvette (vial) before putting it in the tester...finger prints need to be gone!
2. Putting the vial in the tester with liquid on the outside (which could ruin your tester).
So wipe it clean before going into the tester. You don't want to be putting this smudged up, wet vial into your Hanna Checker:

With wet chemistry methods, I am very familiar with API and Red Sea kits, both of which use titration as a means to determine both alkalinity and calcium. I am not aware of any alkalinity or calcium test kit that requires matching the color of the solution to a particular color on a card, typical of API nitrate, nitrite and ammonia kits, just to mention a few. If such color matching kits exist for alkalinity and calcium, I personally would not like them because I have a very difficult time matching the color in the vial to the color on the card. For some reason, when using these color matching kits, the color I get in the vial doesn't match any of the colors on the card. I should point out that I am colorblind, and that might very well be the reason I have difficulty with these kits. With the titration alkalinity and calcium kits—where I'm just looking for a color change—I have no problem.
So API and Red Sea (and others) are simple color changing titration kits, and my preference with these is the Red Sea Pro kit. This Pro kit includes all three test kits in one package; alkalinity, calcium and magnesium kits. This kit simply changes colors when the titration is done.

The common errors I see folks making with these kits are:
1. Not following instructions. Even though I've done these tests hundreds of times, I still pull out the summary card and follow the instructions explicitly. This is especially true with the magnesium test (from Red Sea), where you need to add five drops of a solution...see that little x5 on the card.

2. Thinking they need to match the color on the summary card to the color in the vial. The titration is over when it changes for one color to the other, where one more drop has no effect on the color. It's not like the test kits where you are matching the exact color on the card.
3. Reading the syringe wrong. With Red Sea, the syringe, when full is 1 ml. When you are done with the titration, the amount you put into the vial IS NOT THE READING ON THE SYRINGE. You need to take that reading on the syringe and subtract it from 1. So if the syringe says 0.4, the amount you used is 1 - 0.4 = 0.6 mls.
C. A Video Summary on Two Part
I'm including this video from BRS because I thought they did a good job of summarizing the two part system.
How To: Dosing Two Part in Your Reef Tank
D. So How Much Do I Need
Even though this has been covered in the linked articles and video above, I'm going to summarize my preferences in how to determine the amount of alkalinity and calcium I need to add to my tank on a daily basis.
INITIAL TESTING ----> ADJUST TO IDEAL LEVELS ---> DETERMINE CONSUMPTION ---> BEGIN DOSING
First thing is to test your alkalinity, calcium and magnesium levels and adjust them to where you would like them to be. Recommended levels are calcium around 400ppm, alkalinkity 7 - 11 dKH, and magnesium 1380 - 1400 ppm. When you do your original testing, use a reef calculator to determine how much you'll need to bring up your levels to where you'd wish them to be. I use the BRS calculator found HERE to figure out how much I'll need. Note that with calcium, you don't want to raise more than 50 ppm per day, and with alkalinity I like to raise no more than 1 dKH per day, until I'm where I want to be. When adding alkalinity and calcium, you want to add these in a high flow area, and not at the same time. If you add them at the same time, you'll get immediate precipitation of calcium carbonate. So separate the additions by a couple minutes to allow the solution to disperse.
Once you've gotten your numbers where you want them, test again about 24 hours later and note the drop. If you have an established tank with colonies of hard corals (and clams), you can use this number to determine your daily consumption, and again, using the calculator to determine how much you need to start dosing to maintain those levels. If you only have a few hard corals or just frags, you may wish to test again on day 2 and maybe day 3 to get a better idea of your daily consumption, and now use that number to determine dosing.
I would now recommend daily testing to make fine adjusts to the amounts you are dosing. Note with Magnesium, I do not dose daily. Dependent on the salt you are using, you may only need to dose weekly or monthly with magnesium. Continue daily testing and adjustments until you are confident in the way your tank is consuming alkalinity and calcium. Once stabilized, you can back off on testing to maybe weekly, or whatever time period you feel comfortable doing, knowing your tank numbers aren't moving.
Now sit back and watch your corals grow.
Thank you very imformative. Great article Grego
The subject of diy additives came to mind the other day seeing a large quantity of sodium bicarbonate on amazon.. They advertised '<5ppm heavy metal content'.
And this made me wonder about even just Arm and Hammer.
Have any of you ever sent your systems, or better yet, freshly made SW with some of the additives in it, out for a Triton test?
I know medias are easily introduced, but this made me ponder the sources and their minimal, yet lasting effects.
And this made me wonder about even just Arm and Hammer.
Have any of you ever sent your systems, or better yet, freshly made SW with some of the additives in it, out for a Triton test?
I know medias are easily introduced, but this made me ponder the sources and their minimal, yet lasting effects.
The subject of diy additives came to mind the other day seeing a large quantity of sodium bicarbonate on amazon.. They advertised '<5ppm heavy metal content'.
And this made me wonder about even just Arm and Hammer.
Have any of you ever sent your systems, or better yet, freshly made SW with some of the additives in it, out for a Triton test?
I know medias are easily introduced, but this made me ponder the sources and their minimal, yet lasting effects.
Oh please!
I think you need to immediately contact the FDA and let them know what you've found, and that Arm and Hammer is poisoning all of us with their toxic baking soda.
Last edited:
Well, with my Red Sea Pro kit expiring, and on the advice of @Robthorn above, I just ordered the troika of Salifert test kits.....Alkalinity, Calcium and Magnesium. Having never used these kits, I'm looking forward to giving them a try.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,452
- Reaction score
- 63,845
The subject of diy additives came to mind the other day seeing a large quantity of sodium bicarbonate on amazon.. They advertised '<5ppm heavy metal content'.
And this made me wonder about even just Arm and Hammer.
Have any of you ever sent your systems, or better yet, freshly made SW with some of the additives in it, out for a Triton test?
I know medias are easily introduced, but this made me ponder the sources and their minimal, yet lasting effects.
Arm and Hammer Baking Soda is food grade and has a food grade and USP purity specification. USP has that exact metals specification you mention above.
http://www.armandhammer.com/news/the-power-of-arm-and-hammer-baking-soda.aspx
"The Power of ARM & HAMMER™ Baking Soda
It’s natural.
Baking Soda, also called sodium bicarbonate, is a naturally occurring substance. Our mining and purification process yields “Pure, Safe and Natural” ARM & HAMMER™ Baking Soda. It’s pure enough (more than 99%) to be listed in the US Pharmacopoeia (USP) since 1948.
"
Hopefully you like the Salifert kits. To me they are very easy to use and read.
Keep notes on your test results to compare from test to test. I test alk so much I foget what my last result was all the time.
I am up to 305ml on my alk now. I may have to add a calcium reactor soon.
Keep notes on your test results to compare from test to test. I test alk so much I foget what my last result was all the time.
I am up to 305ml on my alk now. I may have to add a calcium reactor soon.
Hi Paul,
One question if you know the answer, how many grams of Soda Ash is it when you say 2cups of it make 1 Gallons of Alkalinity solution?
What's happening if I put too much Soda Ash in the the RO water? It just stop dissolving?
Thanks
One question if you know the answer, how many grams of Soda Ash is it when you say 2cups of it make 1 Gallons of Alkalinity solution?
What's happening if I put too much Soda Ash in the the RO water? It just stop dissolving?
Thanks
Similar threads
- Replies
- 1
- Views
- 78
-
- AMS: Article
- Replies
- 61
- Views
- 4,210
- Replies
- 8
- Views
- 138
- Replies
- 20
- Views
- 441

















