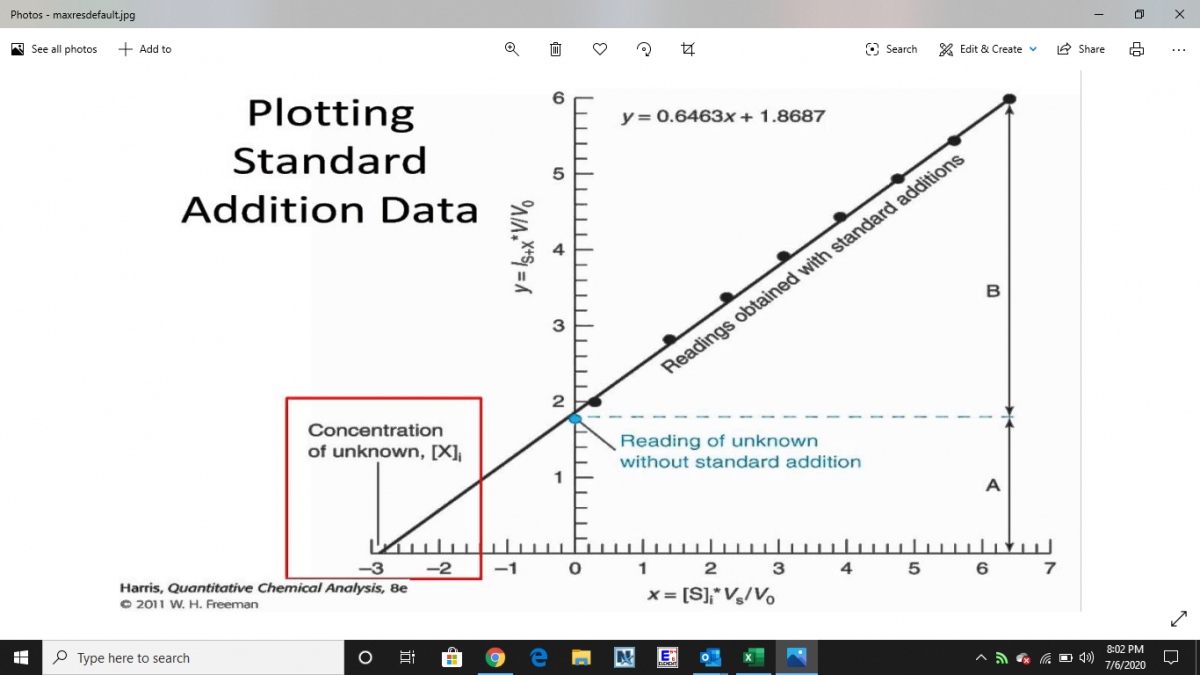
Your aren't missing anything. It's a great question.. and you are right.. it's not a guarantee. When you have to be certain you go to Method of Standard Additions. This method creates a calibration curve with your dirty water that has suspected interference. When you extrapolate the calibration curve you created the line intersects the concentration of the unknown (your water). It is a very labor intensive technique. You would basically make up multiple standards with your reef water and then plot the results in excel. You would then read your reef water result off the curve like below. You can google standard additions and there is a lot of information out there.
Thank for the reply I will ponder this and will most likely have additional questions ....My procedure for evaluation the accuracy as well as precision of an instrument is to create a set of standards within the normal testing range using salt water at my normal salinity made from sodium chloride and RODI water. I then run multiple measurements at each level usually 5 some times as many as 10 if I see a lot of variability in the measurement...I then calculate the relative accuracy and relative precision to get an idea of what error I might expect from the test. I will some times look at the data in a Gage R&R and ANOVA format to get an idea of the components of the error...Instrument, Test protocol, etc. This for the most part assumes the intercept is (0,0) or very close to that....Doesn't always work out that way because of the construction of the meters. @taricha did some good work on characterizing this using drops of vanilla to measure the variability ...