Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,311
- Reaction score
- 63,658
Here's a nice table of some indicator dyes:
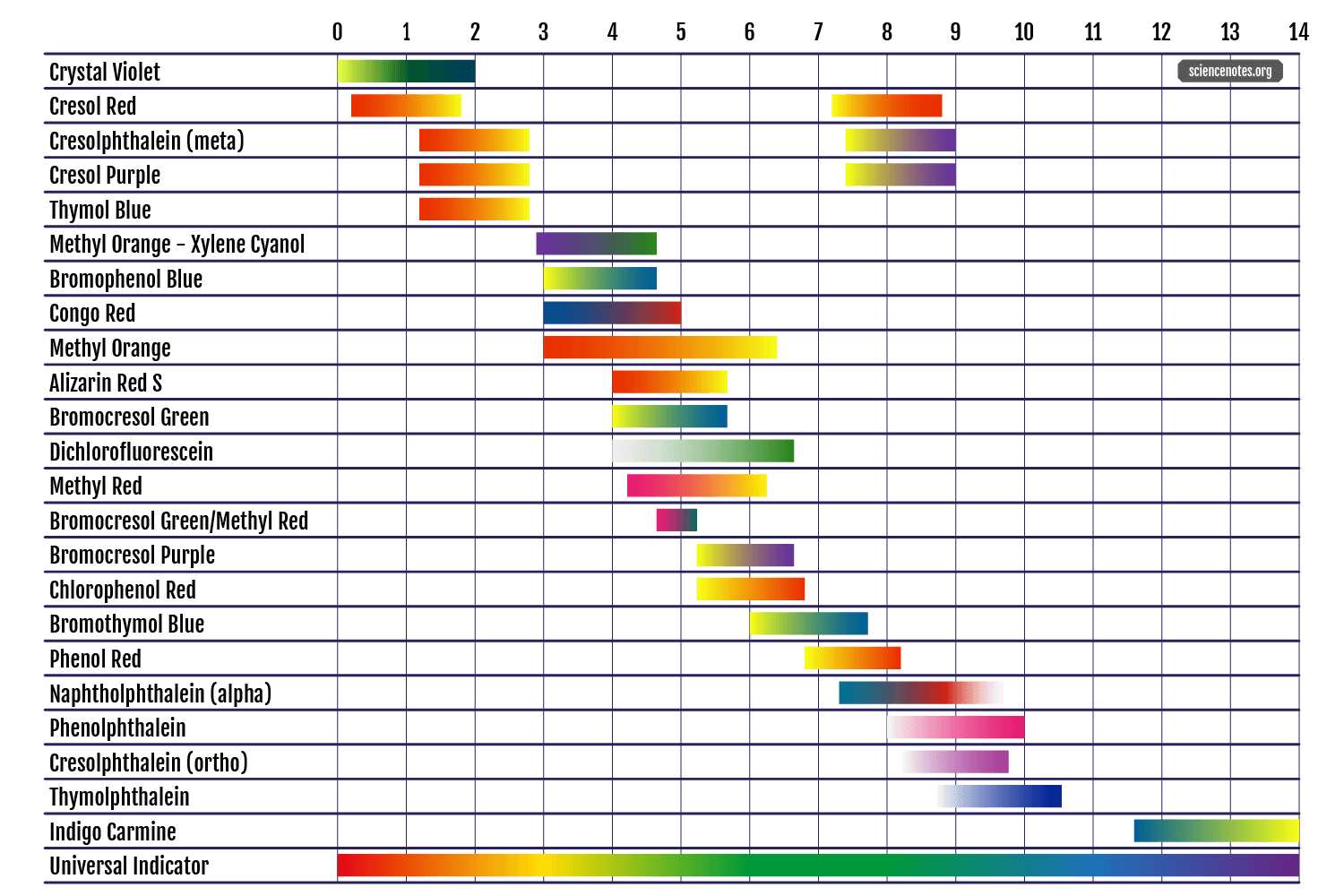
 sciencenotes.org
sciencenotes.org
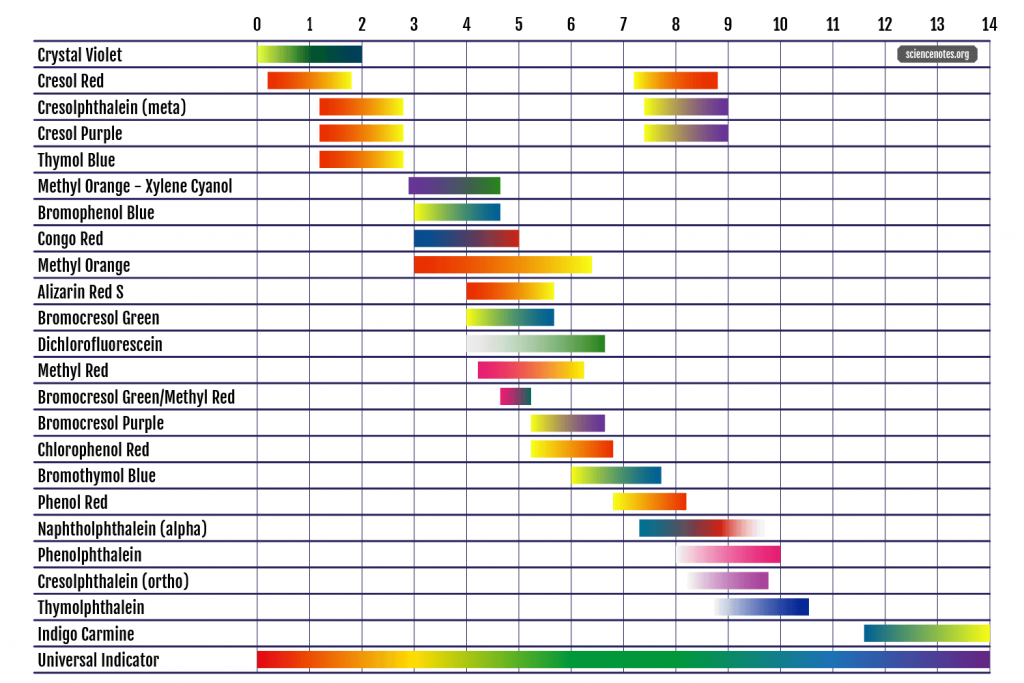

pH Indicator Chart - Colors and Ranges
Get a handy pH indicator chart. See the colors and pH ranges and learn how to choose an acid-base indicator.















