Fellow chemistry inclined reefers,
I'm currently experimenting with Aquavitro Synthesis to raise my nitrates but I believe I'm going to switch to Potassium Nitrate. Upon some research I found this equation (see pictures) to be the general rule of thumb. My problem is I have a 160g system and I DO NOT want to dose 160ml of anything to my tank! So in order to crunch the amounts, I simply divided by 4 to get a more reasonable 40ml dose. My question is, with concentrating the KNo3 itself would I have to increase the amount of RO/DI? Also If so, how much? Even if I use this equation, does it have to be daily? If anyone has a more reliable equation that I can dose possibly more spread out throughout the week, please share. Randy I would really appreciate your input and a possible solid mixing/dosing equation! I included a pic of the simple math I did to get this more concentrated equation, sorry if you can't read my hand writing.


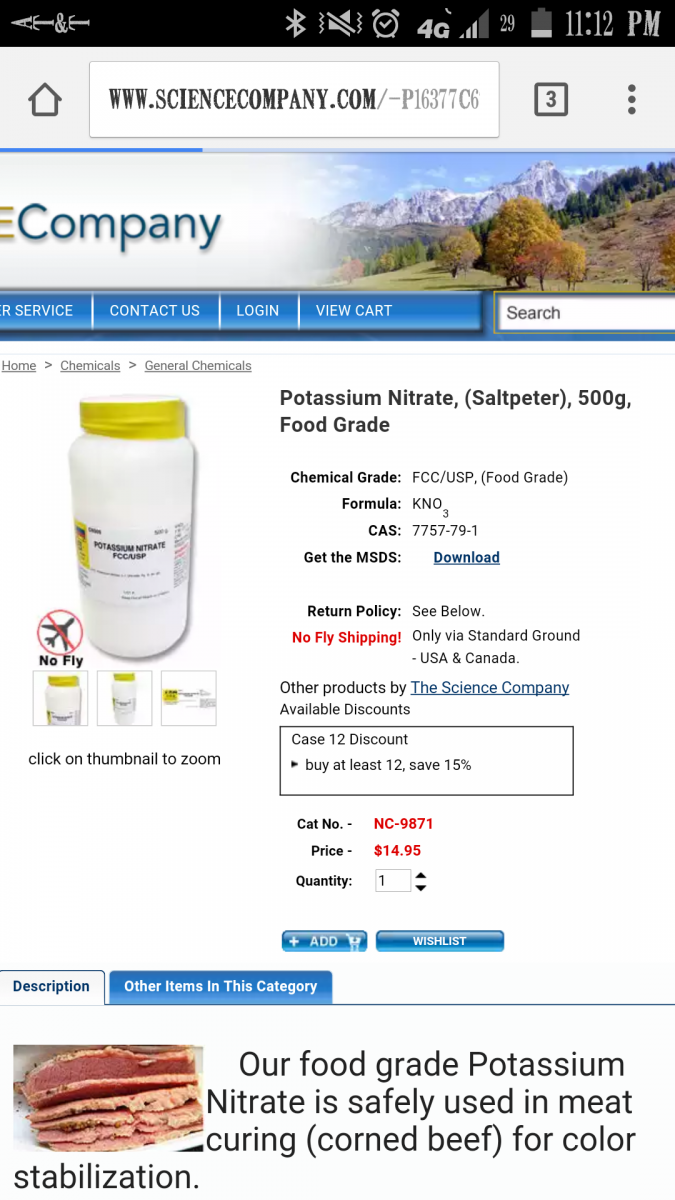
I'm currently experimenting with Aquavitro Synthesis to raise my nitrates but I believe I'm going to switch to Potassium Nitrate. Upon some research I found this equation (see pictures) to be the general rule of thumb. My problem is I have a 160g system and I DO NOT want to dose 160ml of anything to my tank! So in order to crunch the amounts, I simply divided by 4 to get a more reasonable 40ml dose. My question is, with concentrating the KNo3 itself would I have to increase the amount of RO/DI? Also If so, how much? Even if I use this equation, does it have to be daily? If anyone has a more reliable equation that I can dose possibly more spread out throughout the week, please share. Randy I would really appreciate your input and a possible solid mixing/dosing equation! I included a pic of the simple math I did to get this more concentrated equation, sorry if you can't read my hand writing.