Brew12
Electrical Gru
View BadgesExcellence Award
Reef Tank 365
Article Contributor
Moderator Emeritus
North Alabama Reef Club
Article Administrator
My Tank Thread
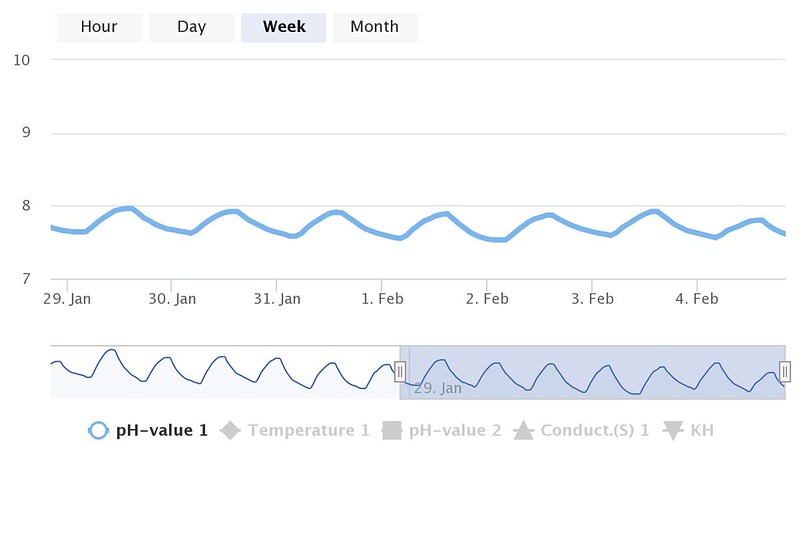
I don't believe that reading is even possible in a reef tank without a constant acid source.I wish it was. I originally started with API, thwn bought a Salifert...same thing. Had a friend come over with his higher end test kit and got a slightly higher reading.