- Joined
- Feb 26, 2017
- Messages
- 70
- Reaction score
- 43
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.





Hi all , does anyone have a up to date recipe for vanadium ?
I’ve got one from some years ago using
“ 99% pure na3v04*12H2o sodium orthovanadate “
Could anyone please help .
After something that I can rig on a doser for constant upkeep .
aswell as have a stock for tweaking
Many thanks Dan

 www.reef2reef.com
www.reef2reef.com
@M5 Dan I use the Vanadium pentoxide (V2O5) 99% from russia hooked up on a doser 0.5ml every 2 days for 50 gallon system and my ICP was spot on.
I think this one got to be the same thing or at least very similar from Amazon UK:
This one is the bottle I purshased from Amazon USA:
My Recipe is base on the one that Randy posted:
Vanadium oxide (V2O5) is 56% vanadium by weight, so dissolving 4gr of it in a Liter of RODI water will result in a concentration of about 2,200 ug/mL.
1 mL of this added to 100 gallons (378.5 L) will boost vanadium by 2,200 ug/mL * 1 mL / 378.5 L = 5.8 ug/L.
That's too high, so if you dilute that stock solution using 100 mL of the previous mix into 1L of RODI then it is 1/10th as much, or 0.6 ug/L final concentration when dosed 1 mL to 100 gallons.
The Vanadium pentoxide is somewhat insoluble on water so to dissolve it you will need to add 1.8 to 2gr of Sodium Hydroxide (caustic soda/Lye) and stir vigorously.
I prefer to use a magnetic stirrer to mix my preparations for 30min, it is much eassier that way and I end by using a disposable coffee filter to strain any impurities that didnt get to mix from the final solution.
Note, the stock solution from the first 4gr of Vanadium last's a very long long time... already a year using it and I haven't used half of the diluted bottle hooked up on the doser. It doesn't seem to go bad or anything, and the bottle of V2O5 powder from russia is gonna last a life time.

I'm looking to fix up my Bromine levels would I use the recipe below?
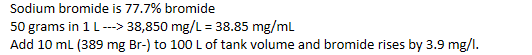
Thank you as always.That sounds reasonable, yes.

The only vanadium I have ever used is V2O5, people are scared of DIY Formulas but to me is working great so far and it is really cheap to make traces on the long-run.Thanks Adrian ,
I will order some of that up .
Have you or do you know if people use sodium orthovanadate for a vanadium recipe ?
I’ve got about 50g of it here an seems a waste of I’m not going to use it but will defo get some pentoxide
@Randy Holmes-FarleyMight be fine too, but I don't see a reason to prefer it.
| Element | Symbol | Mass Percent |
|---|---|---|
| Cobaltum | Co | 38.023% |
| Sulfur | S | 20.688% |
| Oxygenium | O | 41.290% |

@Randy Holmes-Farley
Sorry late to the game on this thread- I ended up getting a lab grade Cobalt Sulfate 99.9% Heptahydrate (CoSO4)
Can you recommend a dilution formula.
Can you double check my logic based on reading this thread.
What is the percentage of CO in CoSO4?
Mass percentage of the elements in the composition
Element Symbol Mass Percent Cobaltum Co 38.023% Sulfur S 20.688% Oxygenium O 41.290%
Do I follow the same rule of thumb as suggested by Reefology1 below but substituting the % by updating the below:
------------------------------------------------------------------------------------------------------------------
Cobalt (II) SulfateHeptahydrate CoSO4 * 7H2O is 38.023% Cobalt by weight
Dissolve 1 gram in 1L of RO/DI. That solution contains 380 mg of cobalt, so the concentration is 380 mg/L or 380 ug/mL.
Adding 1 mL to 100 L of tank water will boost aquarium cobalt levels by 3.8 ug/L.
-----------------------------------------------------------------------------------------------------------------
Thanks, If that is correct general formula can I also apply it for Zinc Chloride (ZnCl2)?
Ellery
Yes here are the cobalt and zinc links:Do you have a link to this product? I get a cobalt weight percentage of 58.9/218.1 = 21%.
