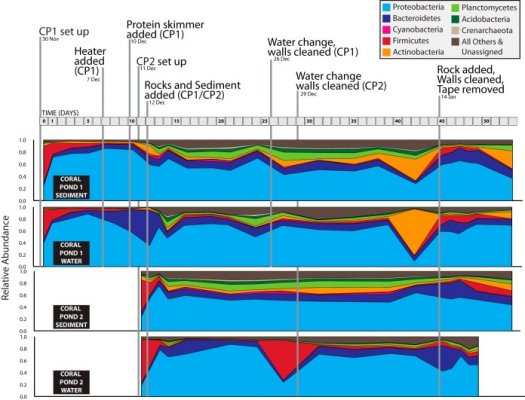
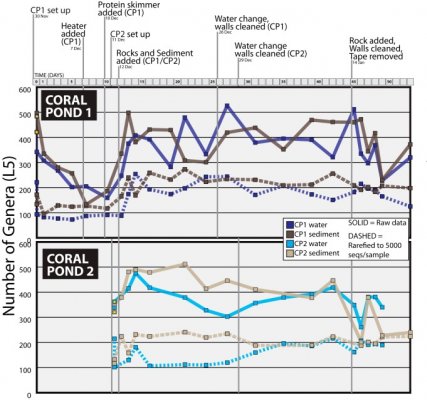
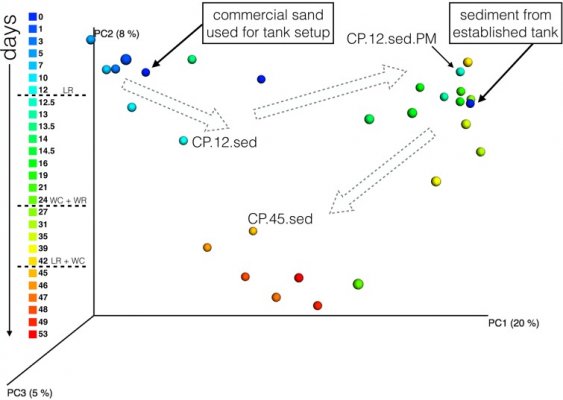
- Joined
- Dec 28, 2016
- Messages
- 22,829
- Reaction score
- 21,964
No - I think you picked out some verbiage that suggested that - but my point was actually - that conventional wisdom about nitrification and ammonia consumption was probably 'voodoo'. This was a scientific article in general about nitrifying bacteria - as compared to nitrifying bacteria in a reef tank. -with no food - for 60 days.Didn’t you list several university studies in the other thread that backed up unassisted cycling