- Joined
- Sep 13, 2018
- Messages
- 360
- Reaction score
- 333
I’ve fought high CO2 levels and pH below 7.6 for years. I’ve taken every approach to raising pH short of a CO2 scrubber. I feel they’re too volatile, have great potential to nuke the tank and can get expensive. Randy’s recent article about pH vs alkalinity stability sparked my experiment . I started dosing lye(NaOH) a couple months ago with the end goal of reaching stable pH AND bicarbonate alkalinity- just like the ocean. Lye is very corrosive, can burn you and nuke your reef without proper PPE, research and redundancy. I’m NOT suggesting everyone go out and start dosing lye. Use kalkwasser, soda ash or esv B-ionic for higher pH first. I’m wanting to demonstrate how you can toggle between these ph boosters and things with minimal impact( carbocalcium, all4reef, bicarbonate) for ultimate stability. In extreme cases like mine, lye dosing can serve as a CO2 scrubber AND supplement the bicarbonate alkalinity your corals use.
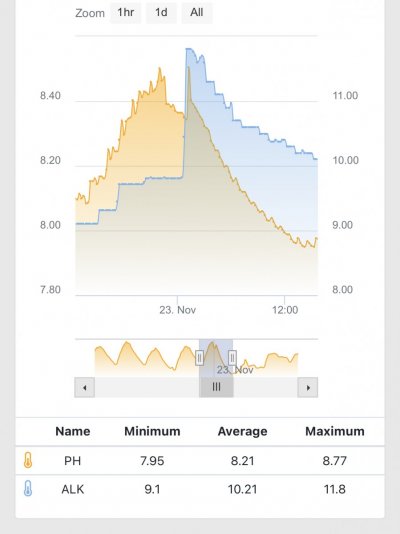
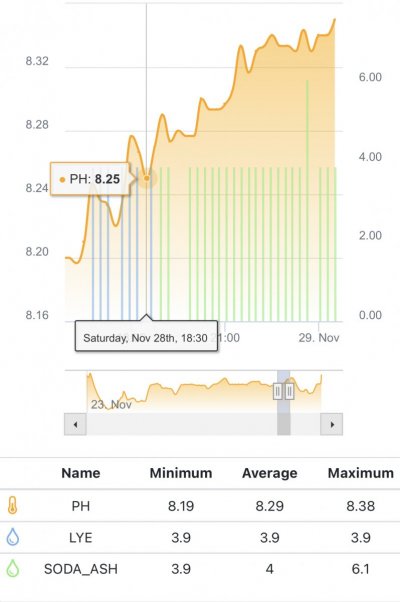
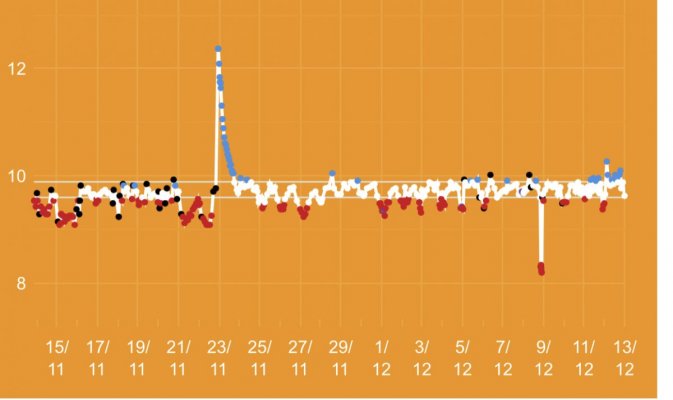
The first 5 weeks were focused on using hydroxide(NaOH and CaOH) for my alkalinity additive chasing only alkalinity stability. Demand became very difficult to keep up with(2.5+dkh/day), pH reached 8.5+ with up to .55 daily swings despite “stable” alk tests. Some corals started showing distress 5 days ago. I presumed the carbonate to bicarbonate ratio was way out of whack. In the name of science, I dumped in a huge 2.5dkh dose of bicarbonate. Corals immediately recovered, alkalinity quickly dropped and stabilized. It was clear the reef needed carbonate additions. Peak demand stripped the reef of CO2 causing the massive pH spikes.
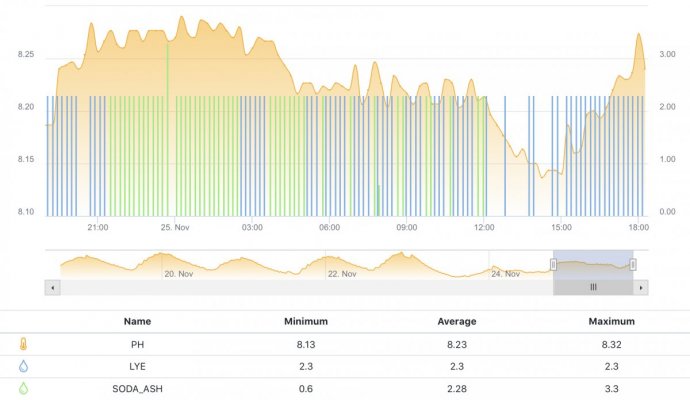
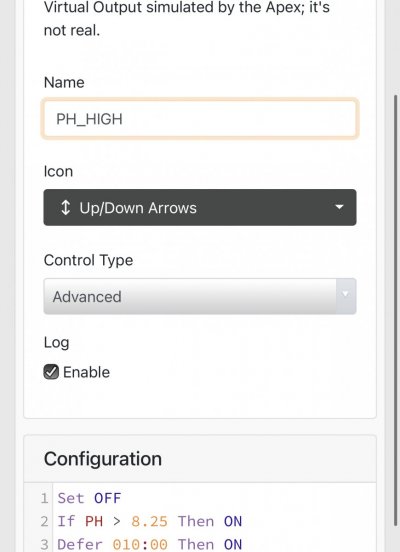
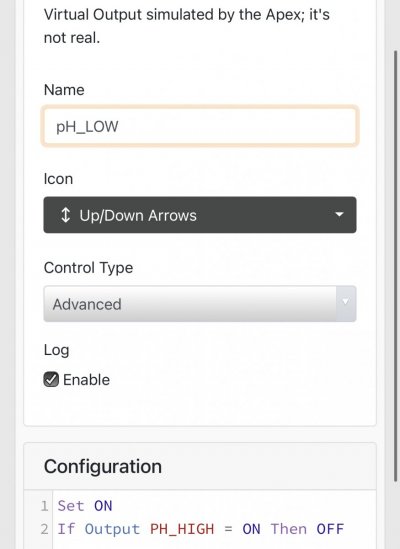
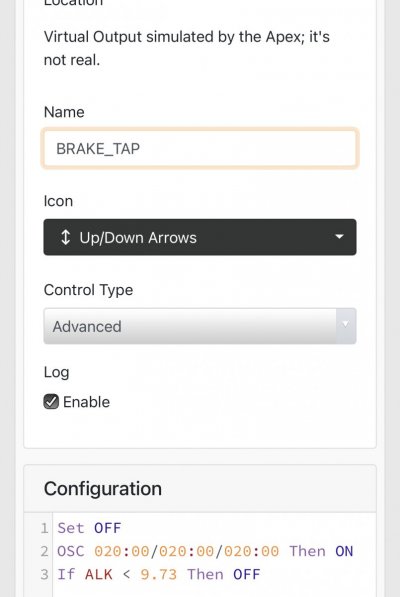
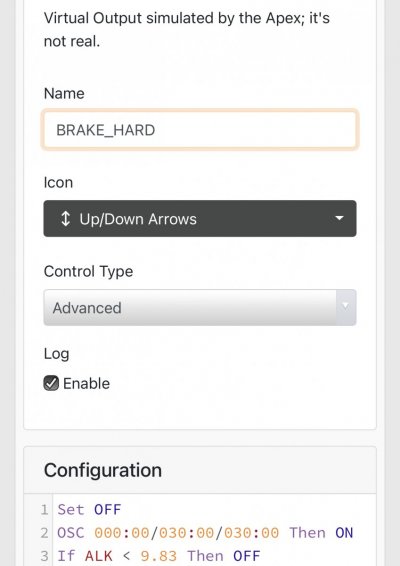
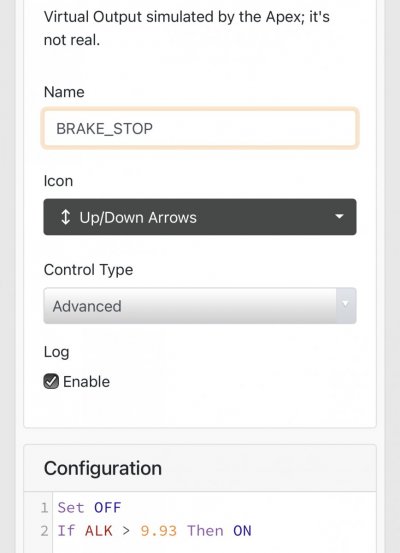
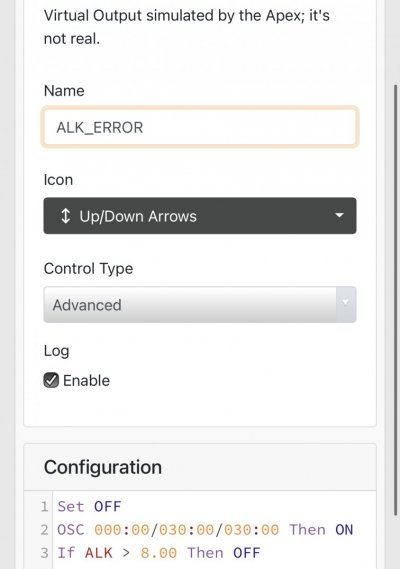
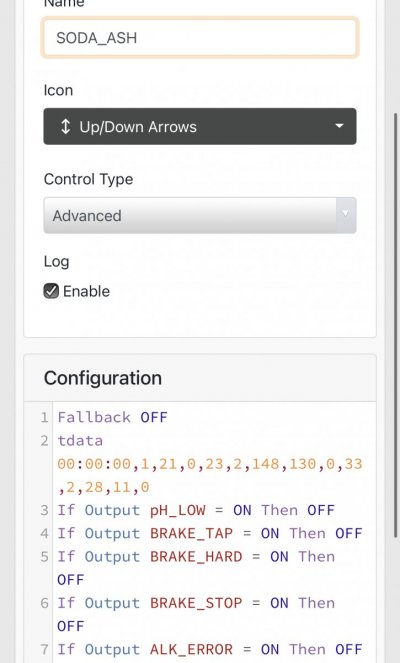
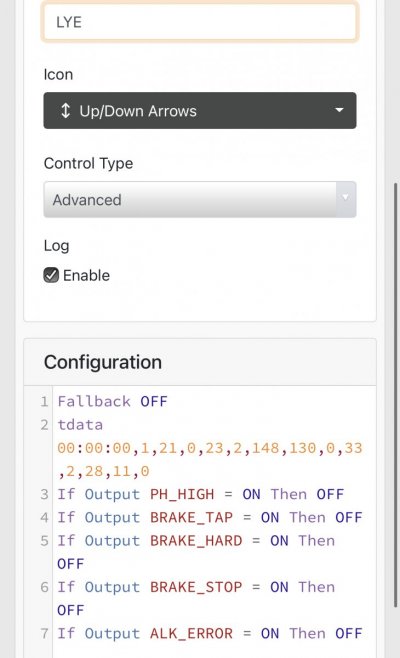
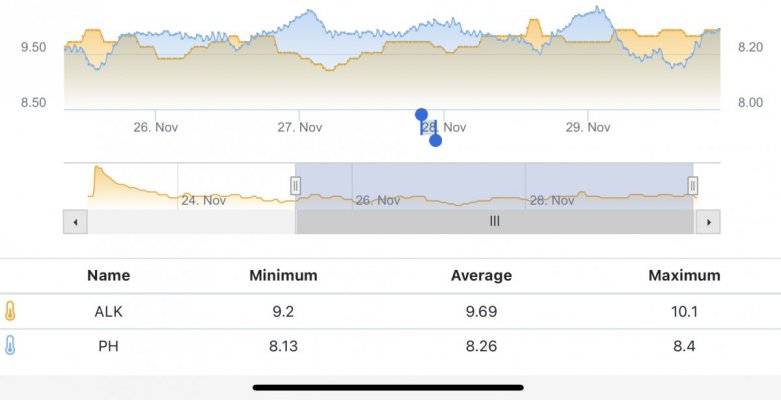
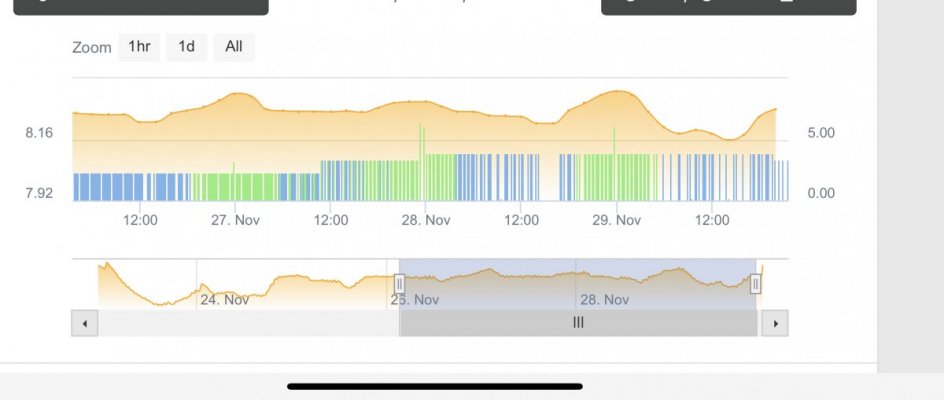
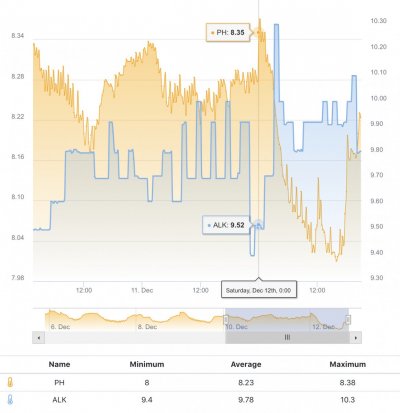
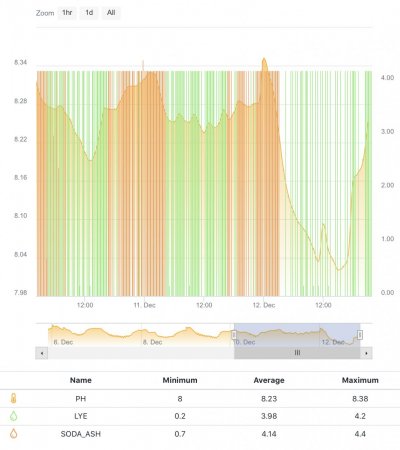
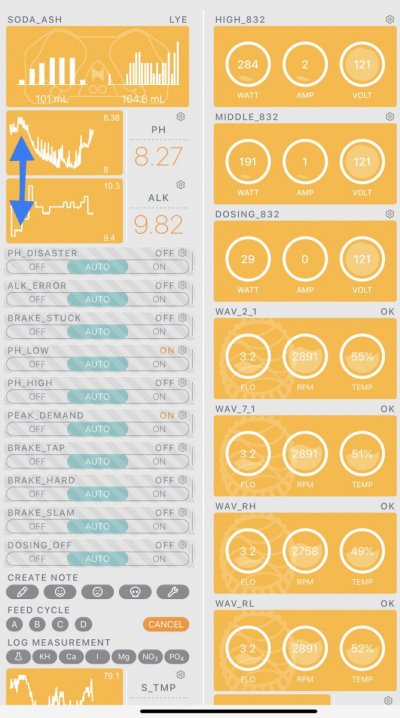
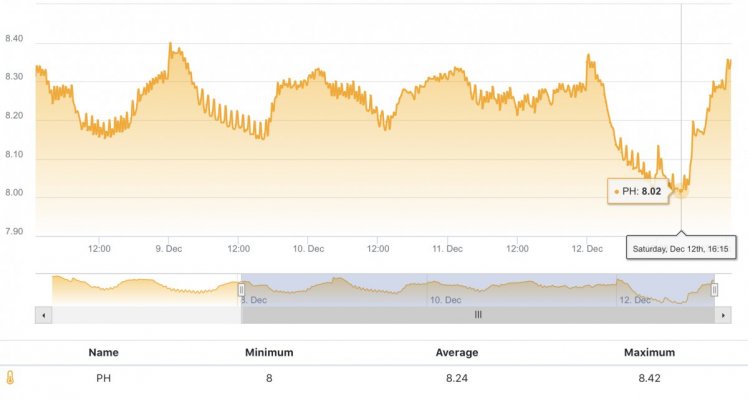
When alk dropped to normal levels I started the final experiment “chasing” alkalinity stability AND pH stability. The Alkatronic tests every 3 hours and hourly forced tests when I’m outside the 9.5-9.9 range. I’m using one dos head for soda ash when pH is above 8.25 and the other with lye when below. My brake_tap VO omits 1/3 of the dose if the Alkatronic sends a 9.8 signal. Brake_hard omits 1/2 the dosing for a 9.9 signal and brake_stop cuts all dosing at 10.0dkh. The goal is to maintain 9.7+/-.2 throughout the day. Toggling between additives is keeping pH high with the smallest swings I’ve ever seen. This is an ongoing experiment and I’m sure I’ll make some minor tweaks. I’ll document the progress here for anyone wanting to follow. I’ll post all the apex code next.
I know just enough about reef chemistry to be dangerous with it. I may be incorrect on certain things and i’d like to hear the experts opinions.
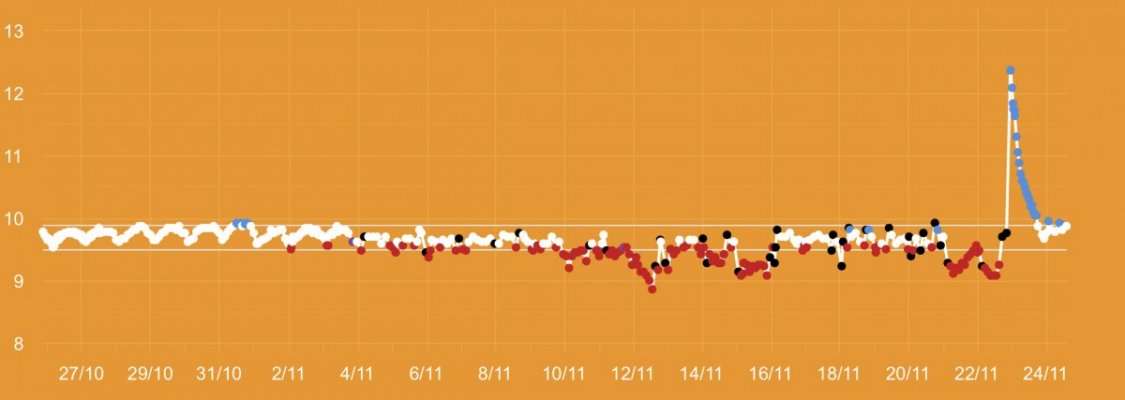
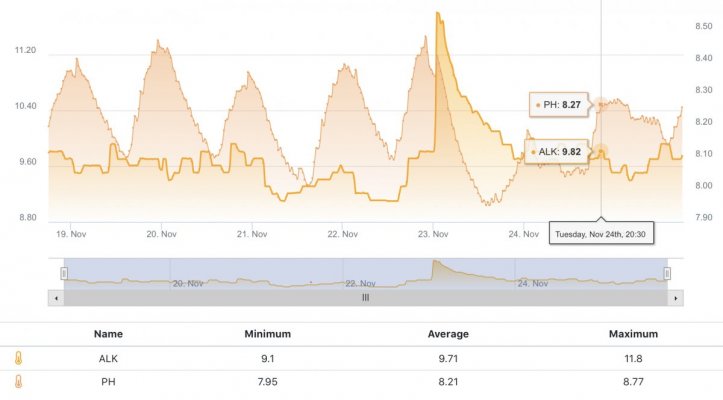
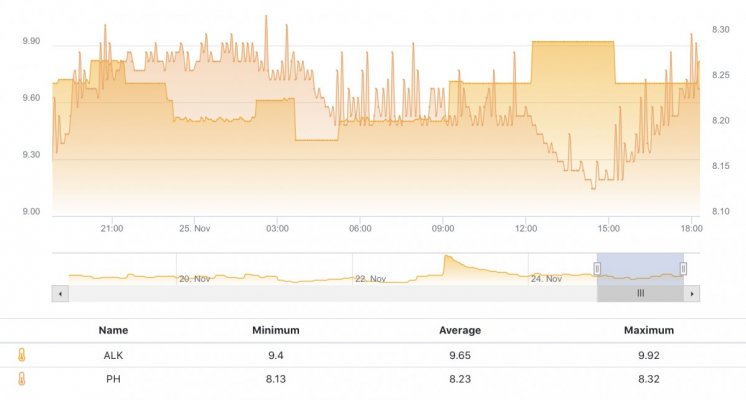
The first 5 weeks were focused on using hydroxide(NaOH and CaOH) for my alkalinity additive chasing only alkalinity stability. Demand became very difficult to keep up with(2.5+dkh/day), pH reached 8.5+ with up to .55 daily swings despite “stable” alk tests. Some corals started showing distress 5 days ago. I presumed the carbonate to bicarbonate ratio was way out of whack. In the name of science, I dumped in a huge 2.5dkh dose of bicarbonate. Corals immediately recovered, alkalinity quickly dropped and stabilized. It was clear the reef needed carbonate additions. Peak demand stripped the reef of CO2 causing the massive pH spikes.
When alk dropped to normal levels I started the final experiment “chasing” alkalinity stability AND pH stability. The Alkatronic tests every 3 hours and hourly forced tests when I’m outside the 9.5-9.9 range. I’m using one dos head for soda ash when pH is above 8.25 and the other with lye when below. My brake_tap VO omits 1/3 of the dose if the Alkatronic sends a 9.8 signal. Brake_hard omits 1/2 the dosing for a 9.9 signal and brake_stop cuts all dosing at 10.0dkh. The goal is to maintain 9.7+/-.2 throughout the day. Toggling between additives is keeping pH high with the smallest swings I’ve ever seen. This is an ongoing experiment and I’m sure I’ll make some minor tweaks. I’ll document the progress here for anyone wanting to follow. I’ll post all the apex code next.
I know just enough about reef chemistry to be dangerous with it. I may be incorrect on certain things and i’d like to hear the experts opinions.