Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,661
- Reaction score
- 64,117
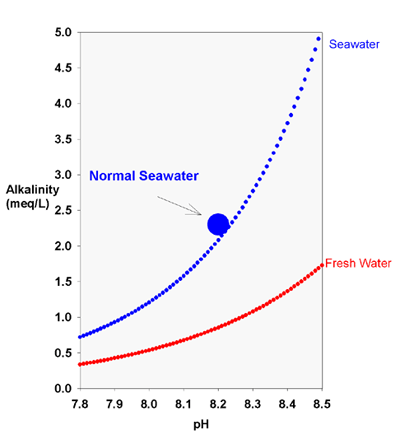
The fact that the expected alk boost from a 0.1 pH unit rise using hydroxide is going to be hard to reliably detect is why I wanted to push the addition at least as high as the directions permit, and is also why I want to test hydroxide doing the same pH boost.