I just started today as well. We'll see how my trend works out. One bit of advice. I mixed to esv concentration. The amount of salts that has to go into part 1 is pretty significant and takes up quite a bit of space. I barely had room in my dosing containers (lexan cereal containers). If you are tight on space you might want to consider larger containers.
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
New DIY Two Part Recipes with Higher pH Boost
- Thread starter Randy Holmes-Farley
- Start date
- Tagged users None
I just started today as well. We'll see how my trend works out. One bit of advice. I mixed to esv concentration. The amount of salts that has to go into part 1 is pretty significant and takes up quite a bit of space. I barely had room in my dosing containers (lexan cereal containers). If you are tight on space you might want to consider larger containers.
Do you mix to result in 1 gallon? or start with 1 gallon and mix the product in? I started with 1/2 gallon or so and then topped off the container to result in 1 gallon total volume.
It's been a few days, so I'll give an initial review of the new dosing solutions. In short, I like them.
They've provided a definite boost to pH as shown below:
As you can see the pH is trending higher, and that's with a house full of dogs and people during the weekend. pH usually trends down with more open mouths in the house on the weekends.
Today's average pH is 8.09. A bump up from last weeks average of 8.02.
Alkalinity initially was holding steady at 7.9 for the first two days. Last night the alkalinity read 7.5 and 7.7, tested twice to confirm. I'm wondering if consumption of alk/cal/mag is increasing due to the slight rise in pH. I'll check alkalinity again tonight and if it's trending downward, I'll go ahead and bump the dosing quantities.
Higher dosing quantity equals more pH boost which equals faster growth, which in turn, requires more dosing? Seems like a good cycle to be in.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,389
- Reaction score
- 63,728
Thanks for the data! 
You're right... I did it wrong. Oops.Do you mix to result in 1 gallon? or start with 1 gallon and mix the product in? I started with 1/2 gallon or so and then topped off the container to result in 1 gallon total volume.
It's been a few days, so I'll give an initial review of the new dosing solutions. In short, I like them.
They've provided a definite boost to pH as shown below:
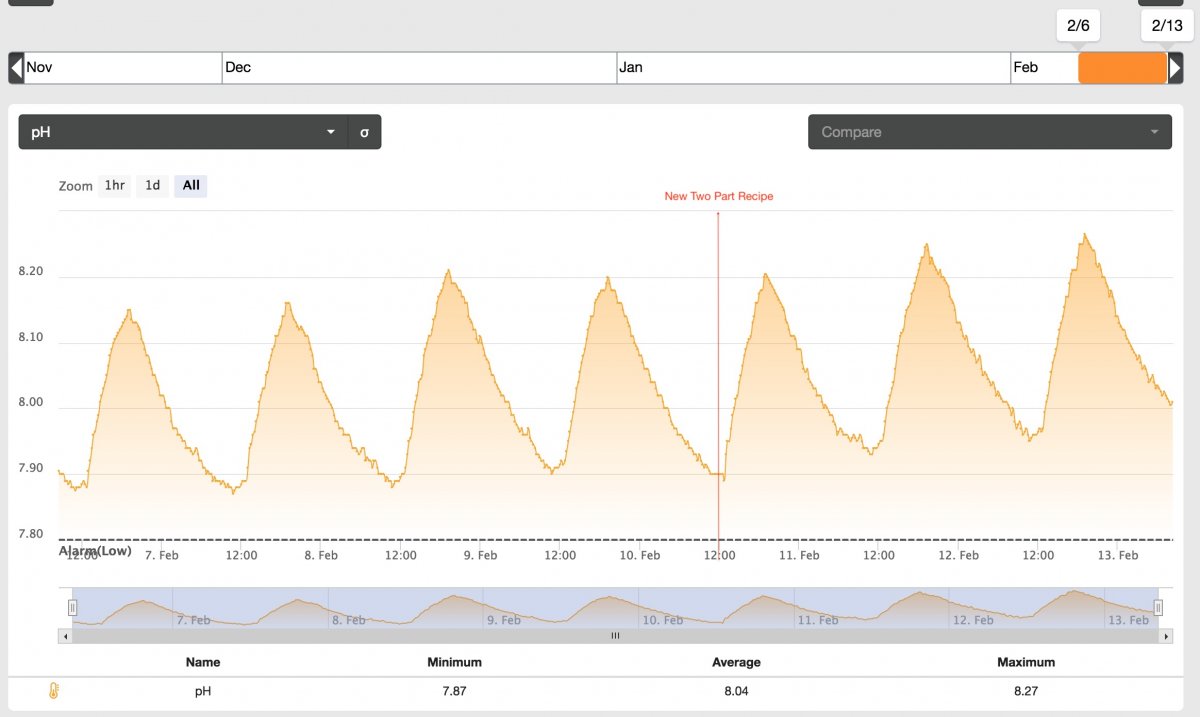
As you can see the pH is trending higher, and that's with a house full of dogs and people during the weekend. pH usually trends down with more open mouths in the house on the weekends.
Today's average pH is 8.09. A bump up from last weeks average of 8.02.
Alkalinity initially was holding steady at 7.9 for the first two days. Last night the alkalinity read 7.5 and 7.7, tested twice to confirm. I'm wondering if consumption of alk/cal/mag is increasing due to the slight rise in pH. I'll check alkalinity again tonight and if it's trending downward, I'll go ahead and bump the dosing quantities.
Higher dosing quantity equals more pH boost which equals faster growth, which in turn, requires more dosing? Seems like a good cycle to be in.
If I wanted to continue to use Kent Tech M independently do I skip the sulfate and just use 282 gram of sodium hydroxide? I just don’t want to goof this up.
You're right... I did it wrong. Oops.
I wasn't sure, but I thought so. Sounds like you'll just have to increase your dosing to match the lower strength.
Actually the strength of the solution should be stronger when mixed correctly vs what I did
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,389
- Reaction score
- 63,728
If I wanted to continue to use Kent Tech M independently do I skip the sulfate and just use 282 gram of sodium hydroxide? I just don’t want to goof this up.
Why would you?
Assuming it is just because you have it already, using it manually is OK, but it does not mitigate the need for sodium sulfate since it only provides enough sulfate (assuming they designed it correctly) to offset the chloride in the magnesium supplement, not the chloride in the calcium chloride.
Unfortunately, you likely cannot use the Tech M directly in this recipe in place of magnesium chloride since it likely isn't compatible in the calcium part due to the sulfate in it.
You could use it in this recipe in place of just the magnesium chloride, and get some Epsom salt (magnesium sulfate heptahydrate) from a drug store:
https://www.reef2reef.com/threads/second-new-diy-two-part-recipe-with-higher-ph-boost.357080/
Hello Randy, and thanks for the post!
Gonna start with this dosing regime, would it be ok if use apex ddr, or i would have a problem due to the high ph of the solution on the acrylic?
And is there any possibility of having the measurements on liters? As in EU is complicated to find gallon bottles
Thanks again!
Gonna start with this dosing regime, would it be ok if use apex ddr, or i would have a problem due to the high ph of the solution on the acrylic?
And is there any possibility of having the measurements on liters? As in EU is complicated to find gallon bottles
Thanks again!
- Joined
- Jun 30, 2017
- Messages
- 452
- Reaction score
- 339
Hello Randy, and thanks for the post!
Gonna start with this dosing regime, would it be ok if use apex ddr, or i would have a problem due to the high ph of the solution on the acrylic?
And is there any possibility of having the measurements on liters? As in EU is complicated to find gallon bottles
Thanks again!
https://www.google.ca/search?q=gall...x-b&gfe_rd=cr&dcr=0&ei=MoKEWqaMGNLM8ged74GABg
LOL! yes i know, but i guess this measurements should be pretty exact...that's why i was asking if there is some with litres
LOL! yes i know, but i guess this measurements should be pretty exact...that's why i was asking if there is some with litres
No, Randy even mentions that you can make it more concentrated, for example. The proportions, however, should be observed. You could use the same recipe and just mix it into 3L. Or 4L. Or cut the quantities in half and mix into 2L.
I just ordered some calcium chloride and magnesium chloride from drs foster smith. It's on sale pretty decent right now approximately 30% off their "house brand". I've never used their brand before, but the dosage recommendations line up with calcium chloride anhydrous and magnesium chloride hexahydrate.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,389
- Reaction score
- 63,728
LOL! yes i know, but i guess this measurements should be pretty exact...that's why i was asking if there is some with litres
If you divide the measurements by 3.79, you get a 1 L recipe with the same potency
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,389
- Reaction score
- 63,728
No, Randy even mentions that you can make it more concentrated, for example. The proportions, however, should be observed. You could use the same recipe and just mix it into 3L. Or 4L. Or cut the quantities in half and mix into 2L.
[emoji106]
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,389
- Reaction score
- 63,728
Since it came up in another thread today...
If you want to add strontium to a DIY two part, here's a rough recipe.
Corals have about 1000 calcium ions for each 9 strontium ions when grown in NSW. Too add about that much to a two part, put in about 1.3 % as much strontium chloride as calcium chloride.
No method can perfectly balance Sr since the demand is about linear with concentration. So your tank will use twice as much Sr (per unit of alk or calcium consumed) at 10 ppm Sr++ as at 5 ppm Sr++.
If you want to add strontium to a DIY two part, here's a rough recipe.
Corals have about 1000 calcium ions for each 9 strontium ions when grown in NSW. Too add about that much to a two part, put in about 1.3 % as much strontium chloride as calcium chloride.
No method can perfectly balance Sr since the demand is about linear with concentration. So your tank will use twice as much Sr (per unit of alk or calcium consumed) at 10 ppm Sr++ as at 5 ppm Sr++.
Since it came up in another thread today...
If you want to add strontium to a DIY two part, here's a rough recipe.
Corals have about 1000 calcium ions for each 9 strontium ions when grown in NSW. Too add about that much to a two part, put in about 1.3 % as much strontium chloride as calcium chloride.
No method can perfectly balance Sr since the demand is about linear with concentration. So your tank will use twice as much Sr (per unit of alk or calcium consumed) at 10 ppm Sr++ as at 5 ppm Sr++.
Would this be an appropriate strontium chloride?
https://www.amazon.com/Science-Company®-NC-2718-Strontium-Chloride/dp/B01M1SP0DV
If so... If we are to use 500g of CaCl2, then we are to add in 6.5g Sr to the CaCl2 mixture?
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,389
- Reaction score
- 63,728
Would this be an appropriate strontium chloride?
https://www.amazon.com/Science-Company®-NC-2718-Strontium-Chloride/dp/B01M1SP0DV
If so... If we are to use 500g of CaCl2, then we are to add in 6.5g Sr to the CaCl2 mixture?
That's likely an OK product to use. You won't likely find food grade as I doubt it gets added to foods.
Note that with the 6 water molecules in that structure, it is only 60% strontium chlorrde by weight.
The 500 grams of calcium chloride you are using is probably the recipe amount in thit thread, which means it is the dihydrate, about 77% calcium chloride.
This 500 grams calcium chloride dihydrate is really about 385 grams of anhydrous calcium chloride.
1.3% of that is 5 grams, which, when corrected for this product being only 60% strontium chloride, you'd use 8.3 grams.
I should have made those issues clearer. Sorry.
That's likely an OK product to use. You won't likely find food grade as I doubt it gets added to foods.
Note that with the 6 water molecules in that structure, it is only 60% strontium chlorrde by weight.
The 500 grams of calcium chloride you are using is probably the recipe amount in thit thread, which means it is the dihydrate, about 77% calcium chloride.
This 500 grams calcium chloride dihydrate is really about 385 grams of anhydrous calcium chloride.
1.3% of that is 5 grams, which, when corrected for this product being only 60% strontium chloride, you'd use 8.3 grams.
I should have made those issues clearer. Sorry.
Thanks. I should have clarified that I was referring to the recipe from this thread.
Side note: Do you have any resources that I could poke around in to help with my general understanding of chemistry? Specifically, when it comes to matters like this, I can follow along with the directions on making the solution, but I struggle with the understanding of, say, how you know that the strontium product I linked has 6 water molecules, or how to determine the amount of anhydrous calcium chloride contained within calcium chloride dihydrate. Thanks again for all your help. I obsessively read over your articles and responses and am continually trying to learn more about the actual processes going on, rather than just what results we'll get from those processes.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,389
- Reaction score
- 63,728
Sure!
The way I knew there were 6 water molecules in the strontium chloride was that the picture of the label shows: SrCl2 6 H2O (blow it up to see that, probably can't see it on a phone)
To get the percentage of pure strontium chloride present, you find the molecular weight of SrCl2 (google it as mw SrCl2; google is a pretty smart chemist; alternatively, add up the molecular weights of all the elements in it). It is 158.5 g/mole. mw is short for molecular weight
Same for SrCl2 6H2O (googling mw SrCl2 6H2O) shows it to be 266.6
So the percentage of SrCl2 in SrCl2 6H2O is 158.5/266.6 = 0.5945 = 59.45% ~60%
I didn't take the starting purity of 95% on the label into account, but you could add 5% more if you want to do that. Problem is it may be a minimum and the actual purity may be higher.
Same way for CaCl2: mw = 111 g/mole
mw ClCl2 2H2O = 147
% CaCl2 --> 111/147 --> ~76% I know that Dowflake always listed it as 77%, so I used that instead since these products may not be fully 2 H2O and I figured Dow knew the "most likely" hydration level.
The way I knew there were 6 water molecules in the strontium chloride was that the picture of the label shows: SrCl2 6 H2O (blow it up to see that, probably can't see it on a phone)
To get the percentage of pure strontium chloride present, you find the molecular weight of SrCl2 (google it as mw SrCl2; google is a pretty smart chemist; alternatively, add up the molecular weights of all the elements in it). It is 158.5 g/mole. mw is short for molecular weight
Same for SrCl2 6H2O (googling mw SrCl2 6H2O) shows it to be 266.6
So the percentage of SrCl2 in SrCl2 6H2O is 158.5/266.6 = 0.5945 = 59.45% ~60%
I didn't take the starting purity of 95% on the label into account, but you could add 5% more if you want to do that. Problem is it may be a minimum and the actual purity may be higher.
Same way for CaCl2: mw = 111 g/mole
mw ClCl2 2H2O = 147
% CaCl2 --> 111/147 --> ~76% I know that Dowflake always listed it as 77%, so I used that instead since these products may not be fully 2 H2O and I figured Dow knew the "most likely" hydration level.
Similar threads
-
- AMS: Article
- Replies
- 61
- Views
- 4,133
- Replies
- 5
- Views
- 422
- Replies
- 8
- Views
- 125
- Replies
- 17
- Views
- 356


















