IMO it can be pretty related and if nitrates are very low while phosphate still detectable it should be discontinued for sure...IMO, it is unrelated to the Redfield ratio, but cyano can take up organic carbon, and if the cyanobacteria is at problem levels, might be a reason to switch to a different carbon source, or stop entirely, yes. It was why I stopped vodka and chose vinegar instead.
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Redfield Ratio Revisited – What are we doing wrong?
- Thread starter Reef and Dive
- Start date
- Tagged users None
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,622
- Reaction score
- 64,078
Attached. I have many more I can add (at work now). These are very nice articles worth reading.
If even one does what you claim, that will prove your point.
Let's pick the first one, and you can clarify why you think it shows that ratios are the critical factor rather than absolute concentrations being important.
I've read it , along with your arrows and highlighting and do not apparently see it the way you do.
Perhaps we need further clarification of what it even means for the ratio to be important.
IMO, it necessarily means that if you start at values of N and P that are yielding good growth, and you increase either N or P further, you get to a "bad" ratio, where something substantial goes wrong.
Is that true in this paper, or any of them? What experiment shows that?
Smith showed that cyanos bloomed frequently in lakes frequently when ratio was lower than 29, while it was rare above this ratio. This occurred in 17 lakes and was recorded over time. When the ratio changed the dominance of cyano population changed as well.If even one does what you claim, that will prove your point.
Let's pick the first one, and you can clarify why you think it shows that ratios are the critical factor rather than absolute concentrations being important.
I've read it , along with your arrows and highlighting and do not apparently see it the way you do.
Perhaps we need further clarification of what it even means for the ratio to be important.
IMO, it necessarily means that if you start at values of N and P that are yielding good growth, and you increase either N or P further, you get to a "bad" ratio, where something substantial goes wrong.
Is that true in this paper, or any of them? What experiment shows that?
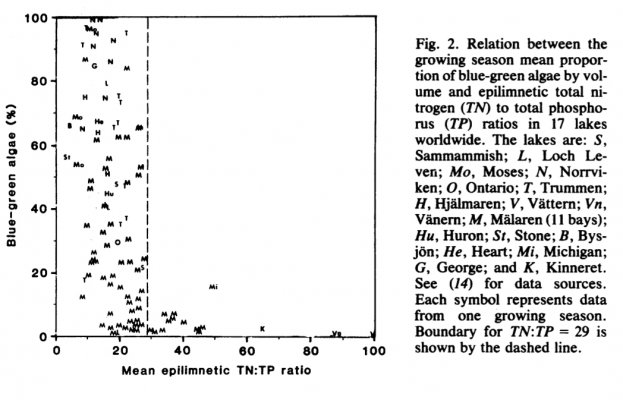
Another example how changing ratios favoured diazotrophsIf even one does what you claim, that will prove your point.
Let's pick the first one, and you can clarify why you think it shows that ratios are the critical factor rather than absolute concentrations being important.
I've read it , along with your arrows and highlighting and do not apparently see it the way you do.
Perhaps we need further clarification of what it even means for the ratio to be important.
IMO, it necessarily means that if you start at values of N and P that are yielding good growth, and you increase either N or P further, you get to a "bad" ratio, where something substantial goes wrong.
Is that true in this paper, or any of them? What experiment shows that?
Attachments
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,622
- Reaction score
- 64,078
I think you misunderstand my point if you think that reference suggests my point is incorrect and yours is correct.
There are very important husbandry differences between my claim and yours, and so it is much more than a scientific or semantic issue.
My point is that all photosynthetic organisms need a sufficient source of N and a sufficient source of P (many more elements too, but these are the point at issue). if either is in short supply, it becomes the limiting nutrient. But if the organism is growing well at a particular availability of N and also of P (let's call this the "good ratio"), then adding more of either N or P has never been shown in any paper I have ever seen to lead to poor growth, regardless of the ratio that is attained. Neither nitrate nor phosphate increases deter organism growth. In that sense, there is no bad ratio, only concentrations that are too low to support growth.
So what about this paper?
It does not say cyano grows faster at lower N : P ratios. It simply says that as a proportion of the organisms present, cyano is higher at lower N : P. NOWHERE does this paper suggest that if you raised N, cyano would grow more slowly.
Of course cyano can prevail over other organisms when N is very low. They say exactly why:
"
The nutrient physiology of the Cyanophyta
differs from that of other algae in
that many blue-green species are capable
of nitrogen fixation. This ability allows
nitrogen-fixing species to maintain high
growth rates in habitats deficient in inorganic
nitrogen, and they should thus be
superior nutrient competitors under conditions
of nitrogen limitation."
and
"
blue-green algae (both
those that fix nitrogen and those that do
not) are generally inferior to diatoms as
phosphorus competitors, indicating that
blue-green algae should typically be
dominant in lakes with low N
which most phytoplankton species
would be nitrogen-limited) and rare in
lakes with high N
Thus, the graph they show is the expected result of cyano being more able to outcompete other species when N is low.
You might contend that is because the ratio is best for cyano. I contend that is because the N concentrations are best for cyano relative to other organisms that cannot fix nitrogen.
Getting back to the important point of husbandry and how these two approaches differ, let's look at dinos.
Many reefers have been plagued by dinos when nutrients are very low. I'm not sure it is clear whether that means low N or low P or both, but it doesn't matter for this point.
My approach is to boost N and P to the point where other organisms (e.g., possible algae or diatoms) thrive and outcompete the dinos for something (space, trace element, etc.). Say, 2-5 ppm nitrate and 0.02-0.1 ppm phosphate.
Your "ratio driven" approach might suggest to increase N or P to some desirable ratio, but it should also include lowering one of N and P to attain the same desirable ratio.
Do you believe that in a dino/low nutrient situation, you can get rid of the dinos by lowering either of N or P to drive the presumably "bad ratio" to a better ratio where something good happens?
Do you believe there is an N : P ratio where dinos stop thriving, that is independent of the actual concentrations of N and P going into that ratio?
Taking a different case, high N and P availability has caused a lot of green algae. Say, 50 ppm nitrate and 0.5 ppm phosphate. In my concentration driven approach, i would contend that unless either of N or P can be driven down to the point where one or both become limiting factors, the actual amounts of N and P present in the water are unimportant and the ratio is unimportant: both are present in sufficient quantities.
In your ratio driven approach, you must contend that adding more of N or P to attain the "good ratio" will cause the algae to recede. Do you actually believe that would succeed? What adjustment to the 50/0.5 ppm scenario would you suggest drives it to a better ratio?
Last edited:
Amazing!I think you misunderstand my point if you think that reference suggests my point is incorrect and yours is correct.
There are very important husbandry differences between my claim and yours, and so it is much more than a scientific or semantic issue.
My point is that all photosynthetic organisms need a sufficient source of N and a sufficient source of P (many more elements too, but these are the point at issue). if either is in short supply, it becomes the limiting nutrient. But if the organism is growing well at a particular availability of N and also of P (let's call this the "good ratio"), then adding more of either N or P has never been shown in any paper I have ever seen to lead to poor growth, regardless of the ratio that is attained. Neither nitrate nor phosphate increases deter organism growth. In that sense, there is no bad ratio, only concentrations that are too low to support growth.
So what about this paper?
It does not say cyano grows faster at lower N : P ratios. It simply says that as a proportion of the organisms present, cyano is higher at lower N : P. NOWHERE does this paper suggest that if you raised N, cyano would grow more slowly.
Of course cyano can prevail over other organisms when N is very low. They say exactly why:
"
The nutrient physiology of the Cyanophyta
differs from that of other algae in
that many blue-green species are capable
of nitrogen fixation. This ability allows
nitrogen-fixing species to maintain high
growth rates in habitats deficient in inorganic
nitrogen, and they should thus be
superior nutrient competitors under conditions
of nitrogen limitation."
and
"
blue-green algae (both
those that fix nitrogen and those that do
not) are generally inferior to diatoms as
phosphorus competitors, indicating that
blue-green algae should typically be
dominant in lakes with low Nratios (in
which most phytoplankton species
would be nitrogen-limited) and rare in
lakes with high Nratios."
Thus, the graph they show is the expected result of cyano being more able to outcompete other species when N is low.
You might contend that is because the ratio is best for cyano. I contend that is because the N concentrations are best for cyano relative to other organisms that cannot fix nitrogen.
Getting back to the important point of husbandry and how these two approaches differ, let's look at dinos.
Many reefers have been plagued by dinos when nutrients are very low. I'm not sure it is clear whether that means low N or low P or both, but it doesn't matter for this point.
My approach is to boost N and P to the point where other organisms (e.g., possible algae or diatoms) thrive and outcompete the dinos for something (space, trace element, etc.). Say, 2-5 ppm nitrate and 0.02-0.1 ppm phosphate.
Your "ratio driven" approach might suggest to increase N or P to some desirable ratio, but it should also include lowering one of N and P to attain the same desirable ratio.
Do you believe that in a dino/low nutrient situation, you can get rid of the dinos by lowering either of N or P to drive the presumably "bad ratio" to a better ratio where something good happens?
Do you believe there is an N : P ratio where dinos stop thriving, that is independent of the actual concentrations of N and P going into that ratio?
Taking a different case, high N and P availability has caused a lot of green algae. Say, 50 ppm nitrate and 0.5 ppm phosphate. In my concentration driven approach, i would contend that unless either of N or P can be driven down to the point where one or both become limiting factors, the actual amounts of N and P present in the water are unimportant and the ratio is unimportant: both are present in sufficient quantities.
In your ratio driven approach, you must contend that adding more of N or P to attain the "good ratio" will cause the algae to recede. Do you actually believe that would succeed? What adjustment to the 50/0.5 ppm scenario would you suggest drives it to a better ratio?
That’s exactly my point with the first text “what are we doing wrong”.
I do not at all advocate “good ratios”.
I advocate that understanding ratio helps comprehend how some microorganisms might prevale...
Many successfull reefers report ratios a lot higher (200:1, 100:1). Many others just keep both N and P very low and are successfull as well.
But when someone faces cyano repeatedly for example, it helps understanding it is capable of using N2 and thriving with low nitrates. I is also capable of using bound phosphate and could get it from rocks or substrate. But if you have a very low nitrate and still moderate high phosphate you have a very fine answer for the reasons of the problem.
Sometimes it is actually dangerous to change ratios: in a new tank, with scarce competition, messing with ratios could actually create a dinoflagellate bloom, since this organism prevales with higher ratios together with diatoms and green algae.
It also helps understand how carbon dosing and other methods might mess with this ratios and often cause blooms.
Last but not least, I prefer calling these ratios “stoichiometric ratios of Nitrogen and Phosphorus”, since the term Redfield actually leads to a very old publication that had many errors, and leads to an idea of “perfect numbers”, while his numbers did not even represent actual oceanic ratios as studied by many recent researchers...
Last edited:
You might contend that is because the ratio is best for cyano. I contend that is because the N concentrations are best for cyano relative to other organisms that cannot fix nitrogen.
I do not believe we can simply keep a niche in our reefs "empty". There will always be microorganisms for that niche (bacteria, diatoms, dinos, cyano, green algae, crysophytes, etc).
A very often repeated system is the one that simply:
1 - favors green algae (higher ratios)
2 - provides a better place for growth (ATS, refugium) OR provides competition and consumption (carbon dosing)
3 - while keeping those numbers down if possible
Do you believe that in a dino/low nutrient situation, you can get rid of the dinos by lowering either of N or P to drive the presumably "bad ratio" to a better ratio where something good happens?
Not exactly to treat dinos, because this will just stimulate a cyano bloom and not remove dinos.
But on the other hand, I would never try to raise this ratio while dinos are present, because this will for sure fuel the problem. By the way, I would raise nutrients in this case, but not the ratio...
50/0.5 ppm
That´s a good ratio, but with very high values. I´ve seen excellent reefs without problems with those numbers, but I would not be satisfied, I would try to lower both.
But let´s say those numbers were 5/0.5 ppm => this is a ratio that strongly favors cyano blooms. I would for sure use a phosphate reduction method (GFO), but not try carbon dosing, as it is near certain that cyano will thrive...
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,622
- Reaction score
- 64,078
I do not believe we can simply keep a niche in our reefs "empty". There will always be microorganisms for that niche (bacteria, diatoms, dinos, cyano, green algae, crysophytes, etc).
A very often repeated system is the one that simply:
1 - favors green algae (higher ratios)
2 - provides a better place for growth (ATS, refugium) OR provides competition and consumption (carbon dosing)
3 - while keeping those numbers down if possible
Not exactly to treat dinos, because this will just stimulate a cyano bloom and not remove dinos.
But on the other hand, I would never try to raise this ratio while dinos are present, because this will for sure fuel the problem. By the way, I would raise nutrients in this case, but not the ratio...
That´s a good ratio, but with very high values. I´ve seen excellent reefs without problems with those numbers, but I would not be satisfied, I would try to lower both.
But let´s say those numbers were 5/0.5 ppm => this is a ratio that strongly favors cyano blooms. I would for sure use a phosphate reduction method (GFO), but not try carbon dosing, as it is near certain that cyano will thrive...
I guess I just do not understand why you focus on the ratio rather than just the absolute values being appropriate or not appropriate.
If something important was a function of the ratio and not the absolute values then I'd certainly agree the ratio was important.
But I'm just not seeing that to be the case.
if I said the N : P was a certain ratio (say, 100:1), and I had a certain problem (say, green algae), would you make a recommendation on which of N or P to raise or lower to attain a "good ratio" without knowing what the absolute values were?
if I said the N : P was a certain ratio (say, 100:1), and I had a certain problem (say, green algae), would you make a recommendation on which of N or P to raise or lower to attain a "good ratio" without knowing what the absolute values were?
For this specific ratio I would suggest just to keep a better place for green algae and keep it growing there (ATS, fuge).
Some special considerations: if the person keeps stony corals, for growth, it is woth trying to keep phosphate under 0.07. For better color on acros it would be worth keeping nitrate under 5.
This helps understanding why some amazing tanks thrive even with values usually considered pretty high (eg Marc Levenston’s - MELEV, some of Sanjay’s)...
@melev @sanjay
Last edited:
No, a cardinal error, very often repeated also in other aquarium publications.My bold and Nitrate is NO3, Phosphate is PO4
... and I do my calculation like this
NO3 molar weight is 14 +3*16 = 62. NO3-N will be 14/62 = 0,225 = 22,5 %
50 ppm NO3 will be 50 * 0.225 = 11.25 ppm as NO3-N
PO4 molar weight is 31+4*16 = 95 P will be 31/95 = 0.326 = 32,6 %
0,91 ppm PO4 will be 0.91*0.326 = 0.296 ppm as PO4-P
11,25/0.296 = 38 N/P ratio
...
Sincerely Lasse
These calculations are based on an incorrect assumption.
Redfield Ratio (RR) N/P should be calculated based on units specified in moles of substance (number of atoms).
A mole is the amount of matter (~6*10^23 atoms)
According to Redfield, N/P = 16/1 means 16 mole N/1 mole P
This is not the same as the mass ratio of these elements!!!
After converting moles to mass we get
1 mole N = 1*14(molar mass N) = 14
1 mole P = 1*31(molar mass P) = 31
So the Mass Ratio (MR) N/P is 14/31 = 0.45
To convert MR to RR you need to perform an operation
MR = 0.45*RR
and vice versa
RR=MR/0.45=MR*2.22
The above calculation "11.25/0.296 = 38 N/P" was made based on numbers representing units of mass.
Redfield uses the mole as the unit of calculation.
Converted to units used by Redfield (RR) it will be this
38*2.22 = 84
So for Redfield, not 38 but N/P ratio = 84.
With best wishes, grandfather Józef from Krakow.
Last edited:
I was not talking about the Redfield ratio here because aquarists seldom calculate in moles. I was talking about rates in ppm N and P. Your right that I did not emphasis this - my mistake However in the following post (#32) I clearly mention that I calculate in ppm
Sincerely Lasse
Sincerely Lasse
Sorry for the newbie but...
In your very first introductory post on this topic, we read:
"Possible applications of the Redfield ratio in the management of the marine aquarium ..."
„...106: 16: 1 carbon : nitrogen : phosphorus ratio...“
„...recognized that a new C: N: P ratio of 106:16:1 was obtained and these are the numbers often referenced in our hobby."
and then a dozen or so more references to this relationship and to that extent.
Then dozens more posts around this very Redfield factor.
In addition, all scientific studies use this factor understood only as a mole and from pure elements C, N and P - not their compounds.
It is therefore incomprehensible to introduce a new coefficient and a complicated method of its calculation, incomparable to those commonly used so far.
You can use not pure N and P, but compounds NO3 and PO4 that are convenient for us to measure, and at the same time units of mass, but it must be very clearly emphasized,
because the ratios calculated in this way are quite different from Redfield.
Then the Tales question comes up in the discussion:
"Is nitrate 50 and phosphate 0.91 a big imbalance?"
and then your answer:
"this is a ratio of 38:1 as NO3-N/P" (I don't know what NO3-N means here?)
and then calculate that 38 is the N/P ratio.
But this number is misleading, it has nothing to do with the Redfield ratio.
To sort it out, I suggest using a generally accepted, easy way to convert the NO3/PO4 ratio into the N/P Redfield ratio:
N/P Redfield = 1.53*NO3/PO4
Now coming back to the discussed question posed by Tales:
"Is nitrate 50 and phosphate 0,91 is a big imbalance?“ we calculate:
NO3/PO4 = 50/0.91 = 54.94
N/P Redfield = 1.53*NO3/PO4 = 1.53*54.94 = 84
so in the Redfield topic, the correct value is a factor of 84 (38 has nothing to compare it to)
However, N/P = 84 : 1 is a big imbalance.
With best wishes, grandfather Józef from Krakow.
In your very first introductory post on this topic, we read:
"Possible applications of the Redfield ratio in the management of the marine aquarium ..."
„...106: 16: 1 carbon : nitrogen : phosphorus ratio...“
„...recognized that a new C: N: P ratio of 106:16:1 was obtained and these are the numbers often referenced in our hobby."
and then a dozen or so more references to this relationship and to that extent.
Then dozens more posts around this very Redfield factor.
In addition, all scientific studies use this factor understood only as a mole and from pure elements C, N and P - not their compounds.
It is therefore incomprehensible to introduce a new coefficient and a complicated method of its calculation, incomparable to those commonly used so far.
You can use not pure N and P, but compounds NO3 and PO4 that are convenient for us to measure, and at the same time units of mass, but it must be very clearly emphasized,
because the ratios calculated in this way are quite different from Redfield.
Then the Tales question comes up in the discussion:
"Is nitrate 50 and phosphate 0.91 a big imbalance?"
and then your answer:
"this is a ratio of 38:1 as NO3-N/P" (I don't know what NO3-N means here?)
and then calculate that 38 is the N/P ratio.
But this number is misleading, it has nothing to do with the Redfield ratio.
To sort it out, I suggest using a generally accepted, easy way to convert the NO3/PO4 ratio into the N/P Redfield ratio:
N/P Redfield = 1.53*NO3/PO4
Now coming back to the discussed question posed by Tales:
"Is nitrate 50 and phosphate 0,91 is a big imbalance?“ we calculate:
NO3/PO4 = 50/0.91 = 54.94
N/P Redfield = 1.53*NO3/PO4 = 1.53*54.94 = 84
so in the Redfield topic, the correct value is a factor of 84 (38 has nothing to compare it to)
However, N/P = 84 : 1 is a big imbalance.
With best wishes, grandfather Józef from Krakow.
Last edited:
This was a very important insight. Previous calculations actually worked better with the lower ratios (eg 5:1). This makes the correct ratio even more predictive.N/P Redfield = 1.53*NO3/PO4
Last edited:
First I have not write the introductory post - its another and they talk about the Redfield ratio. However - I do not think that I mention the Readfield anywhere
Really - and you claim a scientific approach?
I think that I´m not have mentioned the Redfield ration in any of the post I done in that thread and there is a reason for that because Redfield ratio is only valid for oceanic plankton not for life itself, and especially not for closed aquariums.
Yes - it could be missunderstod in some posts
Yes - because Redfield is no valid for aquarium
I´m not comparing to a ratio in open sea
A final conclusion:
What does the Redfield ratio stands for - what its really says is that there is 16 atoms of N one every atom of P. Its a quantity quota. I want to use mass quota instead because it will be easier to calculate if your measurements give you the answer in mass - mg/L (or ppm which is popular in the US but not exact in saltwater) I know that I often have use ppm instead of mg/l but lately I always try to use mg/l instead
The atomic mass for N (in older literature atom weight) is 14 and for P 31 (around). 16*14 N = 224 in total mass for these 16 atoms - the total mass for 1 atom of P is still 31. This means that mass quota will be 224/31 to 1 = 7,23:1. Expressed as mg/L it will be 7,23 mg N /L to 1 mg P/L and it correspond rather good to 7,23:1 in ppm too. If you want to convert this int a NO3/PO4 mass ratio you need to calculate with the molecular weights instead. Now you can express your mass quota of N/P as NO3-N/PO4-P - it will still be 7,23:1
7,23 NO3 have a molecule mass of 7.23* 62 = 448,26 1 PO4 have a molecule mass of 31+64 = 95. The mass quota of NO3/PO4 that respond to the 16 to 1 quantity quota in the Redfield is therefore around 4,72:1 -> 4.72 mg NO3/L to 1mg PO4/L
Sincerely Lasse
Its a typo in that post - the right text should be "this is a ratio of 38:1 as NO3-N/PO4-P" - my badThen the Tales question comes up in the discussion:
"Is nitrate 50 and phosphate 0.91 a big imbalance?"
and then your answer:
"this is a ratio of 38:1 as NO3-N/P"
(I don't know what NO3-N means here?)
Really - and you claim a scientific approach?
No - but I do not say that either -if you read my other posts - I have concentrate on the questions of cyanobacteria and dinoflagellate outbreaks in aquaria. We are measuring our nutrients in ppm (or mg/L) and there can the NO3 molecule be of special interest - at least for the forming of cyanobacteria mats. For us - as aquarists is best to have our ratio in the units we measure - and that´s mass ratio:s expressed as NO3/PO4 or NO3-N/PO4-P.and then calculate that 38 is the N/P ratio.
But this number is misleading, it has nothing to do with the Redfield ratio.
Yes if you work with nutrient concentrations among the planktons in open sea - but we do notTo sort it out, I suggest using a generally accepted, easy way to convert the NO3/PO4 ratio into the N/P Redfield ratio:
I think that I´m not have mentioned the Redfield ration in any of the post I done in that thread and there is a reason for that because Redfield ratio is only valid for oceanic plankton not for life itself, and especially not for closed aquariums.
You can use not pure N and P, but compounds NO3 and PO4 that are convenient for us to measure, and at the same time units of mass, but it must be very clearly emphasized,
Yes - it could be missunderstod in some posts
because the ratios calculated in this way are quite different from Redfield.
Yes - because Redfield is no valid for aquarium
so in the Redfield topic, the correct value is a factor of 84 (38 has nothing to compare it to)
I´m not comparing to a ratio in open sea
A final conclusion:
What does the Redfield ratio stands for - what its really says is that there is 16 atoms of N one every atom of P. Its a quantity quota. I want to use mass quota instead because it will be easier to calculate if your measurements give you the answer in mass - mg/L (or ppm which is popular in the US but not exact in saltwater) I know that I often have use ppm instead of mg/l but lately I always try to use mg/l instead
The atomic mass for N (in older literature atom weight) is 14 and for P 31 (around). 16*14 N = 224 in total mass for these 16 atoms - the total mass for 1 atom of P is still 31. This means that mass quota will be 224/31 to 1 = 7,23:1. Expressed as mg/L it will be 7,23 mg N /L to 1 mg P/L and it correspond rather good to 7,23:1 in ppm too. If you want to convert this int a NO3/PO4 mass ratio you need to calculate with the molecular weights instead. Now you can express your mass quota of N/P as NO3-N/PO4-P - it will still be 7,23:1
7,23 NO3 have a molecule mass of 7.23* 62 = 448,26 1 PO4 have a molecule mass of 31+64 = 95. The mass quota of NO3/PO4 that respond to the 16 to 1 quantity quota in the Redfield is therefore around 4,72:1 -> 4.72 mg NO3/L to 1mg PO4/L
Sincerely Lasse
Last edited:
sixty_reefer
5000 Club Member
View BadgesArticle Contributor
UK Reef Club Member
Hospitality Award
R2R Research
Sorry for the newbie but...
In your very first introductory post on this topic, we read:
"Possible applications of the Redfield ratio in the management of the marine aquarium ..."
„...106: 16: 1 carbon : nitrogen : phosphorus ratio...“
„...recognized that a new C: N: P ratio of 106:16:1 was obtained and these are the numbers often referenced in our hobby."
and then a dozen or so more references to this relationship and to that extent.
Then dozens more posts around this very Redfield factor.
In addition, all scientific studies use this factor understood only as a mole and from pure elements C, N and P - not their compounds.
It is therefore incomprehensible to introduce a new coefficient and a complicated method of its calculation, incomparable to those commonly used so far.
You can use not pure N and P, but compounds NO3 and PO4 that are convenient for us to measure, and at the same time units of mass, but it must be very clearly emphasized,
because the ratios calculated in this way are quite different from Redfield.
Then the Tales question comes up in the discussion:
"Is nitrate 50 and phosphate 0.91 a big imbalance?"
and then your answer:
"this is a ratio of 38:1 as NO3-N/P" (I don't know what NO3-N means here?)
and then calculate that 38 is the N/P ratio.
But this number is misleading, it has nothing to do with the Redfield ratio.
To sort it out, I suggest using a generally accepted, easy way to convert the NO3/PO4 ratio into the N/P Redfield ratio:
N/P Redfield = 1.53*NO3/PO4
Now coming back to the discussed question posed by Tales:
"Is nitrate 50 and phosphate 0,91 is a big imbalance?“ we calculate:
NO3/PO4 = 50/0.91 = 54.94
N/P Redfield = 1.53*NO3/PO4 = 1.53*54.94 = 84
so in the Redfield topic, the correct value is a factor of 84 (38 has nothing to compare it to)
However, N/P = 84 : 1 is a big imbalance.
With best wishes, grandfather Józef from Krakow.
how does different residual between nitrates and phosphates become a imbalance?
wouldn’t the redfield ratio be more well applied to the uptake of nutrients and far more important Vs the residual no3 and po4?
residual definition is: remaining after the greater part or quantity has gone
the mistake we doing is making a ratio with residual wend we should concentrate on implementing the ratio with the uptake, unfortunately the uptake in aquaria is mostly done in the ammonium form and not in nitrates.
Last edited:
how does different residual between nitrates and phosphates become a imbalance?
wouldn’t the redfield ratio be more well applied to the uptake of nutrients and far more important Vs the residual no3 and po4?
residual definition is: remaining after the greater part or quantity has gone
the mistake we doing is making a ratio with residual wend we should concentrate on implementing the ratio with the uptake, unfortunately the uptake in aquaria is mostly done in the ammonium form and not in nitrates.
It is not rocket science actually and pretty easy to understand. Since cyano is very efficient to uptake nitrogen from the air (diazotrophy) it simply gets and advantage when nitrogen is limited in relation to phosphate. It helps understand favourable conditions and why carbon dosing often promotes a cyano bloom.
Tho whole point of the article is to say:
1 - no to look for specific numbers
2 - no to the original publication by Redfield and yes to a lot of information published later
3 - yes to the benefit of comprehension and specific aplications (eg under specific conditions it is pretty easy to solve cyano problems just dosing nitrates; why it does not always work; comprehension of these ratios helps).
And I also recognize there are a lot more factors thar drive algae problems, specially biological, reproduction, competition and sometimes just plain introduction to the system.
I also wanted to provide real scientific evidence that many claims are real since we reefers like to say there is no scientific evidence of things or that scientific evidence is useless because “all systems are different”.
Last edited:
Triton had suggest a quantity quota of 147 N atoms on 1 P atom
It will correspond to a mass quota NO3-N/PO4-N of 147*14 NO3-N -> 2058 NO3-N to 1*31 PO4-N. This will give a quota of 66.4:1 NO3-N/PO4-N (N/P)
Edit
If we instead calculate with mass quota of NO3/PO4 we will have 66.41*62/95 -> 43.3;1 as mass quota for NO3/PO4.
It means - if you read 0.1mg/L PO4 - you should aim for around 4 - 5 mg/L NO3, If you read 0.05 mg/L PO4 - corresponding NO3 is between 2 an3 mg/L
This is wrong - my bad - right text would be as ithis
"My calculation of the Triton NO3/PO4 mass quota is therefore as wrong - the right mass quota according Triton would be 147NO3/1PO4 = 95.9:1 - with other words the same mass quota as @jm48 calculated in another way - my bad"
This is also not exactly right either but not as wrong as the text above
Interesting is that most aquarists with good working aquarium just reports figures like this as more or less the golden spot.
For what it is worth - last PO4 I took was around 0.7 mg/L and my NO3 was around 23 mg/L - which give me a mass quota of 32.9 - in fact rather close to Tritons figures - my aquarium is among those High nutrient aquariums that run fine - exactly as Thales do - and if we recalculate that into mass quota of NO3/PO4 we get 50/0.9 -> around 55:1 not so far away from Triton either
Sincerely Lasse
It will correspond to a mass quota NO3-N/PO4-N of 147*14 NO3-N -> 2058 NO3-N to 1*31 PO4-N. This will give a quota of 66.4:1 NO3-N/PO4-N (N/P)
Edit
If we instead calculate with mass quota of NO3/PO4 we will have 66.41*62/95 -> 43.3;1 as mass quota for NO3/PO4.
It means - if you read 0.1mg/L PO4 - you should aim for around 4 - 5 mg/L NO3, If you read 0.05 mg/L PO4 - corresponding NO3 is between 2 an3 mg/L
This is wrong - my bad - right text would be as ithis
"My calculation of the Triton NO3/PO4 mass quota is therefore as wrong - the right mass quota according Triton would be 147NO3/1PO4 = 95.9:1 - with other words the same mass quota as @jm48 calculated in another way - my bad"
This is also not exactly right either but not as wrong as the text above
For what it is worth - last PO4 I took was around 0.7 mg/L and my NO3 was around 23 mg/L - which give me a mass quota of 32.9 - in fact rather close to Tritons figures - my aquarium is among those High nutrient aquariums that run fine - exactly as Thales do - and if we recalculate that into mass quota of NO3/PO4 we get 50/0.9 -> around 55:1 not so far away from Triton either
Sincerely Lasse
Last edited:
Well, it won't 7,23:1!!!Now you can express your mass quota of N/P as NO3-N/PO4-P - it will still be 7,23:1
7,23 NO3 have a molecule mass of 7.23* 62 = 448,26 1 PO4 have a molecule mass of 31+64 = 95. The mass quota of NO3/PO4 that respond to the 16 to 1 quantity quota in the Redfield is therefore around 4,72:1 -> 4.72 mg NO3/L to 1mg PO4/L
Sincerely Lasse
There is a fundamental logical error here.
It still can't be 7.23:1 because there are additional O3 and O4 in the compounds, and oxygen (O) has different molar masses than N and P.
Quantitative proportions cannot be transferred to mass proportions if the same amount of atom has different masses.
If this is difficult for someone to understand, here's an example:
N -> Nectarine = 14g
P -> Papaya = 31 g
O -> Orange = 16g
16* Nectarine / 1* Papaya = 16*14/1*31 = 7.23 Approx. But not anymore.
We make basket 1 (16*NO3), its mass is:
16*(1*Nectarine + 3*Orange) = 16*(1*14+3*16) = 992g
We make basket 2 (PO4), its mass is:
1*Papaya + 4*Orange = 1*31+ 4*16 = 95g
We calculate the mass ratio
basket 1 (16*NO3) / basket 2 (PO4) = 992/ 95 = 10.44 !!!
As you can see, this is not equal to 7.23 ?
And now I come back to what I presented earlier, how easy it is to convert the N/P atom (mol) ratio to the NO3/PO4 mass ratio (mg/l), it will always be:
N/P (atom) = 1.53*NO3/PO4 (mg/l)
All the above and previous calculations for which Lasse can be thrown into the bin are based on incorrect assumptions - you cannot mix Nectarine, Papaya and Orange in this way. The result for Triton 43.3:1 as a mass fraction for NO3/PO4 is similarly grossly wrong.Triton had suggest a quantity quota of 147 N atoms on 1 P atom
It will correspond to a mass quota NO3-N/PO4-N of 147*14 NO3-N -> 2058 NO3-N to 1*31 PO4-N. This will give a quota of 66.4:1 NO3-N/PO4-N (N/P)
If we instead calculate with mass quota of NO3/PO4 we will have 66.41*62/95 -> 43.3;1 as mass quota for NO3/PO4.
It should be correct 147:1 = 1.53* 96:1
N/P(atom) = 147:1 corresponds to NO3/PO4 = 96:1 (mg/l)
If you don't believe me, please recalculate using Nectarine, Papaya and Orange
I never suggested it, but 9974 messages don't mean that either.Really - and you claim a scientific approach?
With best wishes, grandfather Józef from Krakow.
It seems that you still not understand what the expression NO3-N and PO4-P stands for. Its stand for the N respectively P portion of the NO3 resp PO4 molecule. NO3-N is the same as N but the expressions says that this N atom comes from the molecule NO3 and PO4-P stands for the P portion of PO4. NO3-N:O4-P is the same as N/P in this case
In your example NectarineOrange3 - Nectarine stands for the Nectarine part of the basket - weight 14 g
PapayaOrange4 - Papaya stands for the Papaya part of the basket - weight 31 g
16 NectarineOrange3 - Nectarine stands for the Nectarine part of the basket - weight 16*14 g = 224 g
1 PapayaOrange4 - Papaya stands for the Papaya part of the basket - weight 31 g
Mass quota 16 NectarineOrange3 - Nectarine/1 PapayaOrange4 - Papaya will therefore be 7.23
Now to my apologize - I must have got a smaller cerebral haemorrhage last night - the calculation for the mass quota of NO3/PO4 was total wrong, NO3 has 1 N atom and PO4 has 1 P atom. If we want 16:1 in atoms we should calculate the quota between 16NO3 and 1 PO4 (it will give us a quantum quota of 16:1 N/P or NO3-N/PO4-P)
The right calculation will be 16*62/95 = 10.44. The same as @jm48 calculate in an other way. My bad. The awkward situation is that I in many other post on this subject - in other threads - I always have use this mass quota (10.44:1) for Redfield ration of NO3/PO4 but did not react by myself of the wrong calculation - but it was late night
My calculation of the Triton NO3/PO4 mass quota is therefore as wrong as my calculation above - if all N species would be NO3-N the right mass quota according Triton would be 147NO3/1PO4 = 95.9:1 - with other words the same mass quota as @jm48 calculated in another way - my bad
But back to this last text in post 98 and Triton N/P quantity quota
I´m very sorry for my posts 97 and 98 - they was not up to my normal standard - they was as wrong as I could see it by myself (and that´s really bad )
)
But my principal standpoint in this we discuss in this thread stands still, Redfield ratio is not a good tool in aquarium but Tritons quantity quota transferred to a NO3/PO4 ratio can be helpful. My expiriences - in my aquarium (and only my aquarium) says that a mass quota NO3/PO4 of around 30 works well and this is rather alike the suggested Triton quantity quota of total N/total P of 147:1 One explanation - total P in this calculation assumes that all P is of the form PO4-P. In most aquariums where P is taken by ICP and PO4 analyse using photometry, PO4-P and total P are very close. Even in my aquarium, which normally contains quite a lot of particles, it has been shown that total P and PO4-P are normally very close to each other. The figure shows analyses done by Oceamo in Austria (they incorporate photometric PO4 analysis in their classic ICP analyse package)
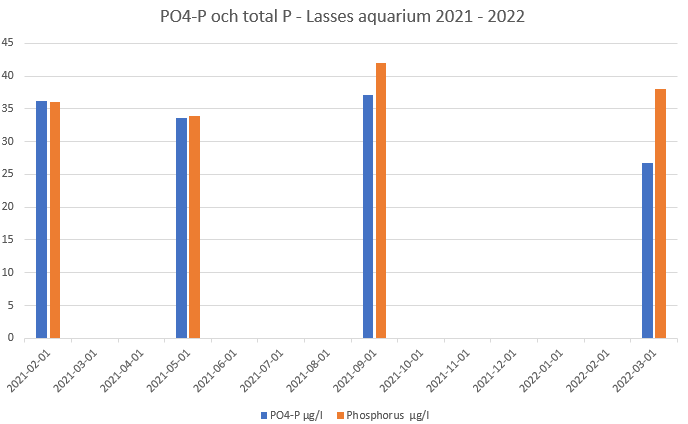
I hope I got it right this time
Sincerely Lasse
In your example NectarineOrange3 - Nectarine stands for the Nectarine part of the basket - weight 14 g
PapayaOrange4 - Papaya stands for the Papaya part of the basket - weight 31 g
16 NectarineOrange3 - Nectarine stands for the Nectarine part of the basket - weight 16*14 g = 224 g
1 PapayaOrange4 - Papaya stands for the Papaya part of the basket - weight 31 g
Mass quota 16 NectarineOrange3 - Nectarine/1 PapayaOrange4 - Papaya will therefore be 7.23
Now to my apologize - I must have got a smaller cerebral haemorrhage last night - the calculation for the mass quota of NO3/PO4 was total wrong, NO3 has 1 N atom and PO4 has 1 P atom. If we want 16:1 in atoms we should calculate the quota between 16NO3 and 1 PO4 (it will give us a quantum quota of 16:1 N/P or NO3-N/PO4-P)
The right calculation will be 16*62/95 = 10.44. The same as @jm48 calculate in an other way. My bad. The awkward situation is that I in many other post on this subject - in other threads - I always have use this mass quota (10.44:1) for Redfield ration of NO3/PO4 but did not react by myself of the wrong calculation - but it was late night
My calculation of the Triton NO3/PO4 mass quota is therefore as wrong as my calculation above - if all N species would be NO3-N the right mass quota according Triton would be 147NO3/1PO4 = 95.9:1 - with other words the same mass quota as @jm48 calculated in another way - my bad
But back to this last text in post 98 and Triton N/P quantity quota
IMO - its not as wrong as it looks like. Triton calculate their 147/1 quantity quota as total N/P quota - with other worlds they take with NO3-N, NO2-N, NH3-N, NH4-N and all organic-N in the figure 147 with other words total N. I have done a lot of Triton N-DOC analyses there the N in these analyses are total N. When I have compared this with my own analysis of NO3-N - the total N in my aquarium normal is 2 time my NO3-N which should correspond to a NO3/PO4 mass quota of around 48*62/95 -> 31:1 NO3/PO4 - it means that in my last case with 0.7 mg/L PO4 - I should have around 21 mg/L NO3 if the aquarium running good. Yes it is running good and my last PO4 and NO3 was in this county,For what it is worth - last PO4 I took was around 0.7 mg/L and my NO3 was around 23 mg/L - which give me a mass quota of 32.9 - in fact rather close to Tritons figures - my aquarium is among those High nutrient aquariums that run fine - exactly as Thales do - and if we recalculate that into mass quota of NO3/PO4 we get 50/0.9 -> around 55:1 not so far away from Triton either
I´m very sorry for my posts 97 and 98 - they was not up to my normal standard - they was as wrong as I could see it by myself (and that´s really bad
But my principal standpoint in this we discuss in this thread stands still, Redfield ratio is not a good tool in aquarium but Tritons quantity quota transferred to a NO3/PO4 ratio can be helpful. My expiriences - in my aquarium (and only my aquarium) says that a mass quota NO3/PO4 of around 30 works well and this is rather alike the suggested Triton quantity quota of total N/total P of 147:1 One explanation - total P in this calculation assumes that all P is of the form PO4-P. In most aquariums where P is taken by ICP and PO4 analyse using photometry, PO4-P and total P are very close. Even in my aquarium, which normally contains quite a lot of particles, it has been shown that total P and PO4-P are normally very close to each other. The figure shows analyses done by Oceamo in Austria (they incorporate photometric PO4 analysis in their classic ICP analyse package)
Sincerely Lasse
Similar threads
- Replies
- 31
- Views
- 744
-
- Poll
- Replies
- 51
- Views
- 3,455
- Replies
- 28
- Views
- 1,508
















