where did you find cultures of those species to start your own? do they have special requirements or are they similiar to culturing nanno and tetra?I have a 120 g SPS-based system and I have been adding around 300 ml of live phytoplankton either daily or each two-three days for several months. The phyto species were Synechococcus and Phaeodactylum cornutum (both cultured with the Easy Reefs modified formula of F2). I have not observed an increase in either nitrates or phosphates. On the contrary, there is a reduction of phosphate.
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
The decomposing phytoplankton and how can we possibly benefit from it in a Reef.
- Thread starter sixty_reefer
- Start date
- Tagged users None
sixty_reefer
5000 Club Member
View BadgesArticle Contributor
UK Reef Club Member
Hospitality Award
R2R Research
I’ve got it figured it out:
will challenge anyone on here to prove me wrong.
the main issues for different results it’s definitely in the formula of the fertiliser. Most Phytoplankton in the ocean comes in a ratio of 106 : 16 : 1 C : N : P as per redfield ratio, this is a very similar ratio as sea water absorb this component. ( there for As phytoplankton breaks down, Nitrogen and phosphate are released back into the water column and only carbon left.
how does this translate to our tanks:
it all starts in our cultures of phytoplankton, if we use a formula that is high in phosphate will mean that our phytoplankton will absorb a higher amount of phosphate that eventually will be released into our water column.
for example a culture that uses fertiliser in a 1:1 ratio will release nitrogen and phosphate into the water column on a 1:1 ratio if it’s made on a 16:1 ratio N and P it will be release on a 16:1 ratio (similar ratio to the bacteria consumption/transform).
carbon won’t be utilised in the tank and it will build up in the tank (sand bed) or removed via skimming and other filters.
meaning that in my test were dead phytoplankton was used, in time only carbon will be left. Nitrogen and phosphorus will be utilised by beneficial bacteria and tank inhabitants.
I can confirm now that what we see in this video is nitrogen being released back to the atmosphere by bacteria breaking down the phytoplankton.
My above statement will be probably the best connection of implementation of redfield to aquaria.
Note: to make a perfect culture of phytoplankton that will mimic the ocean we need a 16:1 Nitrogen to phosphate ratio.
@taricha does this makes sense to you?
will challenge anyone on here to prove me wrong.
the main issues for different results it’s definitely in the formula of the fertiliser. Most Phytoplankton in the ocean comes in a ratio of 106 : 16 : 1 C : N : P as per redfield ratio, this is a very similar ratio as sea water absorb this component. ( there for As phytoplankton breaks down, Nitrogen and phosphate are released back into the water column and only carbon left.
how does this translate to our tanks:
it all starts in our cultures of phytoplankton, if we use a formula that is high in phosphate will mean that our phytoplankton will absorb a higher amount of phosphate that eventually will be released into our water column.
for example a culture that uses fertiliser in a 1:1 ratio will release nitrogen and phosphate into the water column on a 1:1 ratio if it’s made on a 16:1 ratio N and P it will be release on a 16:1 ratio (similar ratio to the bacteria consumption/transform).
carbon won’t be utilised in the tank and it will build up in the tank (sand bed) or removed via skimming and other filters.
meaning that in my test were dead phytoplankton was used, in time only carbon will be left. Nitrogen and phosphorus will be utilised by beneficial bacteria and tank inhabitants.
I can confirm now that what we see in this video is nitrogen being released back to the atmosphere by bacteria breaking down the phytoplankton.
My above statement will be probably the best connection of implementation of redfield to aquaria.
Note: to make a perfect culture of phytoplankton that will mimic the ocean we need a 16:1 Nitrogen to phosphate ratio.
@taricha does this makes sense to you?
sixty_reefer
5000 Club Member
View BadgesArticle Contributor
UK Reef Club Member
Hospitality Award
R2R Research
The only difference will be that by using dead fresh phytoplankton in a reactor you will end up with all the carbon that a tank won’t be able to process in a vessel that it’s easy to remove and clean instead of relying on filtration for the process. This will avoid it also for it not to be stored in your sand bed as it would happen in the ocean.While I appreciate the effort, I'm still confused on how dosing the dead phyto, is any different then dosing many of the dead phyto products available on the market?
I've dosed live phyto to my tank for many months nows, and I for one will always do it. Used to dose the dead stuff, but all that did was contribute to nitrates and phosphates fueling algae growth.
If dosing enough(I'm up to 2.5ml/G), would not the unused portions end up dying and decomposing in the tank anyways?
Seriously interested in this as like I said, I have dosed dead phyto for years, but only recently( a few months now) started dosing the live stuff, and there is a night and day difference in my tank with the live.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,391
- Reaction score
- 63,730
The only difference will be that by using dead fresh phytoplankton in a reactor you will end up with all the carbon that a tank won’t be able to process in a vessel that it’s easy to remove and clean instead of relying on filtration for the process. This will avoid it also for it not to be stored in your sand bed as it would happen in the ocean.
Two giant issues with this idea:
1. The comments about carbon is incorrect. Reef tanks produce and use massive amounts of carbon, both organic and inorganic.
2. The carbon in phyto is present as organic molecules. The N and P in phyto is also present as organic molecules. A dead phyto organism will release all of these to the water and they will escape a reactor.
sixty_reefer
5000 Club Member
View BadgesArticle Contributor
UK Reef Club Member
Hospitality Award
R2R Research
This kind of support my tough, that the demineralisation of phytoplankton will be released into the water column and the part that is not used would be left behind in the reactor. A quick google would probably show plenty of articles related to climate change and how the carbon from phytoplankton (one of the biggest absorber of co2 in our planet) that is not used gets stored at the bottom of the ocean.Two giant issues with this idea:
1. The comments about carbon is incorrect. Reef tanks produce and use massive amounts of carbon, both organic and inorganic.
2. The carbon in phyto is present as organic molecules. The N and P in phyto is also present as organic molecules. A dead phyto organism will release all of these to the water and they will escape a reactor.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,391
- Reaction score
- 63,730
This kind of support my tough, that the demineralisation of phytoplankton will be released into the water column and the part that is not used would be left behind in the reactor. A quick google would probably show plenty of articles related to climate change and how the carbon from phytoplankton (one of the biggest absorber of co2 in our planet) that is not used gets stored at the bottom of the ocean.
Yes, I'm well aware of the processes involved. Mineralization means formation of calcium carbonate. That not a process that will happen from dead phyto in a reef tank reactor, nor is it one that would be beneficial for a reef tank since it will deplete alkalinity and calcium and accomplish nothing desirable.
sixty_reefer
5000 Club Member
View BadgesArticle Contributor
UK Reef Club Member
Hospitality Award
R2R Research
I meant “remineralisation” typing fast and auto correct doesn’t go well together.Yes, I'm well aware of the processes involved. Mineralization means formation of calcium carbonate. That not a process that will happen from dead phyto in a reef tank reactor, nor is it one that would be beneficial for a reef tank since it will deplete alkalinity and calcium and accomplish nothing desirable.
similar to this
BioLINCS | Nitrogen Cycling in the Open Ocean
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,391
- Reaction score
- 63,730
I meant “remineralisation” typing fast and auto correct doesn’t go well together.
similar to this
BioLINCS | Nitrogen Cycling in the Open Ocean
hahana.soest.hawaii.edu
There's no process that is going to deposit carbon and not a similar amount of N and P in a dead phyto reactor, and I'm not seeing any reason why one would want to.
There's no big problem with organic carbon from foods in a reef tank. it is likely quite desirable, hence the reason why I dosed organic carbon.
where do you buy this from?@elysics
@sixty_reefer
I use to use the Mercer of Montanas f/2 fertilizer but found that it was loaded with undesirable Phosphates.
Standard F/2 (Guillard's) medium compositions (from researchgate.net)
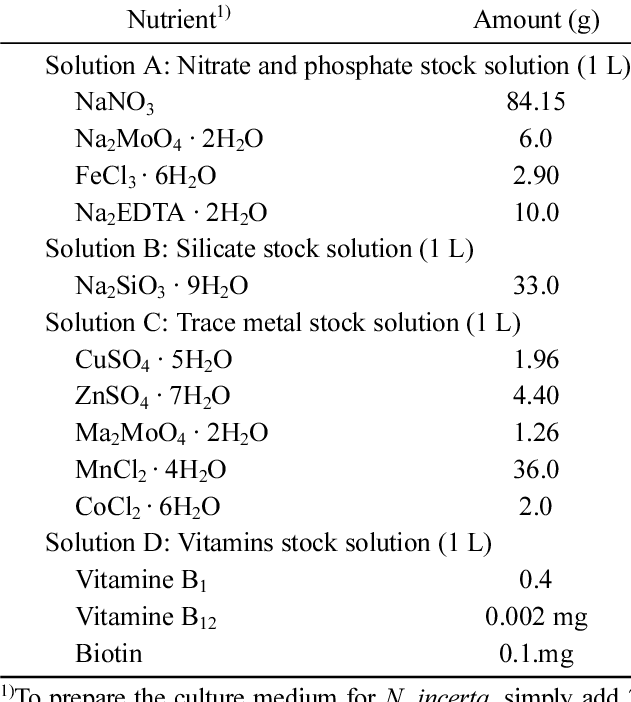
Ive since moved over to using pure lab grade sodium nitrate powder/pellets to culture a new batch of phyto every 2 weeks.

I found that it takes about twice as long to get to the dark green state I desire, but it does get there... without the use of any added Phosphates
So inside my container is 1.020 saltwater (new IO mix), phyto (tetraselmis from previous culture) and pure sodium nitrate.
If Phosphate appears in a batch, it didn't come from me or an outside source I purposefully put in.
.
Sodium nitrate? Amazonwhere do you buy this from?
I’ve got it figured it out:
will challenge anyone on here to prove me wrong.
the main issues for different results it’s definitely in the formula of the fertiliser. Most Phytoplankton in the ocean comes in a ratio of 106 : 16 : 1 C : N : P as per redfield ratio, this is a very similar ratio as sea water absorb this component. ( there for As phytoplankton breaks down, Nitrogen and phosphate are released back into the water column and only carbon left.
how does this translate to our tanks:
it all starts in our cultures of phytoplankton, if we use a formula that is high in phosphate will mean that our phytoplankton will absorb a higher amount of phosphate that eventually will be released into our water column.
for example a culture that uses fertiliser in a 1:1 ratio will release nitrogen and phosphate into the water column on a 1:1 ratio if it’s made on a 16:1 ratio N and P it will be release on a 16:1 ratio (similar ratio to the bacteria consumption/transform).
carbon won’t be utilised in the tank and it will build up in the tank (sand bed) or removed via skimming and other filters.
meaning that in my test were dead phytoplankton was used, in time only carbon will be left. Nitrogen and phosphorus will be utilised by beneficial bacteria and tank inhabitants.
I can confirm now that what we see in this video is nitrogen being released back to the atmosphere by bacteria breaking down the phytoplankton.
My above statement will be probably the best connection of implementation of redfield to aquaria.
Note: to make a perfect culture of phytoplankton that will mimic the ocean we need a 16:1 Nitrogen to phosphate ratio.
@taricha does this makes sense to you?
so do you think dosing phyto to my tank will help with nuisance algae? not sure it will since nitrate and phosphate will be released back into the water
sixty_reefer
5000 Club Member
View BadgesArticle Contributor
UK Reef Club Member
Hospitality Award
R2R Research
Probably would feed on it, it’s hard to tell, I haven’t experienced it myself wend dosing phytoplankton so wouldn’t be able to tell you, it’s tough that some pest algaes will prefer ammonia to nitrates.so do you think dosing phyto to my tank will help with nuisance algae? not sure it will since nitrate and phosphate will be released back into the water
Last edited:
sixty_reefer
5000 Club Member
View BadgesArticle Contributor
UK Reef Club Member
Hospitality Award
R2R Research
2 day update
there is a vast amount of nitrogen bubbles in the phytoplankton, this gives me a clue that the bacteria in the water is rapidly settling in the reactor. There was some gas bubbles in there yesterday just not as much as today.
in the DT still not much improvement on the photosynthetic dinoflagellates and Cyanobacteria. Will leave the sand untouched for a few weeks to see if any differences arise. It seems weaker on the corners maybe still early to insinuate anything.


there is a vast amount of nitrogen bubbles in the phytoplankton, this gives me a clue that the bacteria in the water is rapidly settling in the reactor. There was some gas bubbles in there yesterday just not as much as today.
in the DT still not much improvement on the photosynthetic dinoflagellates and Cyanobacteria. Will leave the sand untouched for a few weeks to see if any differences arise. It seems weaker on the corners maybe still early to insinuate anything.
sixty_reefer
5000 Club Member
View BadgesArticle Contributor
UK Reef Club Member
Hospitality Award
R2R Research
First evidence of the phytoplankton reactor lowering no3. (Green Carbon dosing)
Tonight I’ve run a series of hobbie levels test kits to try and compare the tank water to the effluent from the reactor
The results were very promising to say that we only on day 2.
nh4 on the effluent of the reactor was 0.1 higher than the tank water
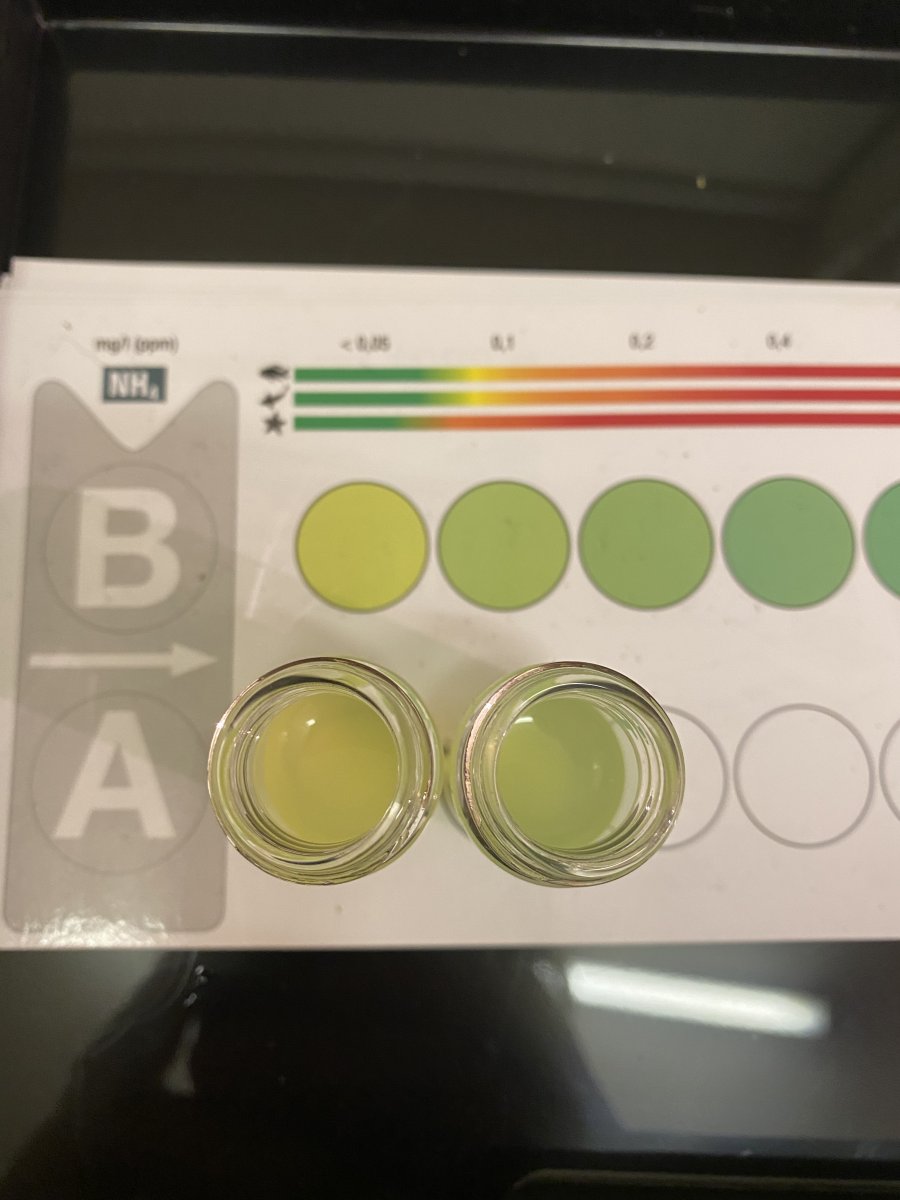
And no3 was lower 5 to 10 ppm than the tank water. (I’ve tested 3 times to be sure)

The only other difference that I noticed was that the effluent from the reactor was 0.5 Dkh Lower than the main tank.
( not sure why KH is 0.5 lower, probably will need to figure it out myself in due time, only thing I can think of at this point is that some of the stored co2 in phytoplankton may be getting released also)
Tonight I’ve run a series of hobbie levels test kits to try and compare the tank water to the effluent from the reactor
The results were very promising to say that we only on day 2.
nh4 on the effluent of the reactor was 0.1 higher than the tank water
And no3 was lower 5 to 10 ppm than the tank water. (I’ve tested 3 times to be sure)
The only other difference that I noticed was that the effluent from the reactor was 0.5 Dkh Lower than the main tank.
( not sure why KH is 0.5 lower, probably will need to figure it out myself in due time, only thing I can think of at this point is that some of the stored co2 in phytoplankton may be getting released also)
sixty_reefer
5000 Club Member
View BadgesArticle Contributor
UK Reef Club Member
Hospitality Award
R2R Research
@Randy Holmes-Farley would this results be acceptable as preliminary evidence that there is unknown benefits? Or is this widely known with most foodsFirst evidence of the phytoplankton reactor lowering no3. (Green Carbon dosing)
Tonight I’ve run a series of hobbie levels test kits to try and compare the tank water to the effluent from the reactor
The results were very promising to say that we only on day 2.
nh4 on the effluent of the reactor was 0.1 higher than the tank water

And no3 was lower 5 to 10 ppm than the tank water. (I’ve tested 3 times to be sure)

The only other difference that I noticed was that the effluent from the reactor was 0.5 Dkh Lower than the main tank.
( not sure why KH is 0.5 lower, probably will need to figure it out myself in due time, only thing I can think of at this point is that some of the stored co2 in phytoplankton may be getting released also)
I have good friends microbiologists in the Spanish universities. Both microorganisms grow well just with the modified F/2. However, adding some drops of a vitamin concentrate (I use Aquaforest) helps to improve the nutritional quality.where did you find cultures of those species to start your own? do they have special requirements or are they similiar to culturing nanno and tetra?
sixty_reefer
5000 Club Member
View BadgesArticle Contributor
UK Reef Club Member
Hospitality Award
R2R Research
This thread continues here if anyone interested

 www.reef2reef.com
www.reef2reef.com

The Green Carbon Dosing Metalology (phytoplankton)
This thread is aimed at discussing the potential of using Phytoplankton as a carbon dosing method and possibly fixing microbiology in a salt water tank during the process. The concept that led me to think that phytoplankton can be beneficial to a reef comes from nature, this is a process that...
 www.reef2reef.com
www.reef2reef.com
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,391
- Reaction score
- 63,730
@Randy Holmes-Farley would this results be acceptable as preliminary evidence that there is unknown benefits? Or is this widely known with most foods
The summary of your observations are
1. There may be a little ammonia in the effluent
2. Nitrate in the effluent looks lower
3. The alkalinity in the effluent is lower
The reactor has what in it?
sixty_reefer
5000 Club Member
View BadgesArticle Contributor
UK Reef Club Member
Hospitality Award
R2R Research
Just freeze dried phytoplankton (20ml according with the spoon provided) and bacteria from the tankThe summary of your observations are
1. There may be a little ammonia in the effluent
2. Nitrate in the effluent looks lower
3. The alkalinity in the effluent is lower
The reactor has what in it?
Similar threads
- Shipping Available
- Replies
- 2
- Views
- 118
- Price: $9-65
- Shipping Available
- Replies
- 2
- Views
- 153
- Replies
- 3
- Views
- 159
- Replies
- 7
- Views
- 779


















