Seems UV can dissipate ozone and products exist that combine ozone using a reactor. Therefore if using UV post ozone with enough distance allowing ozone to perform its function does one still need GAC other than to perform other functions like pulling items not affected by either ozone or UV? Wanting to confirm UV fully neutralizes ozone. I believe it does with hydrogen peroxide but ozone in this manner seems simpler and safer to me.
Illustration of combo I’m considering.
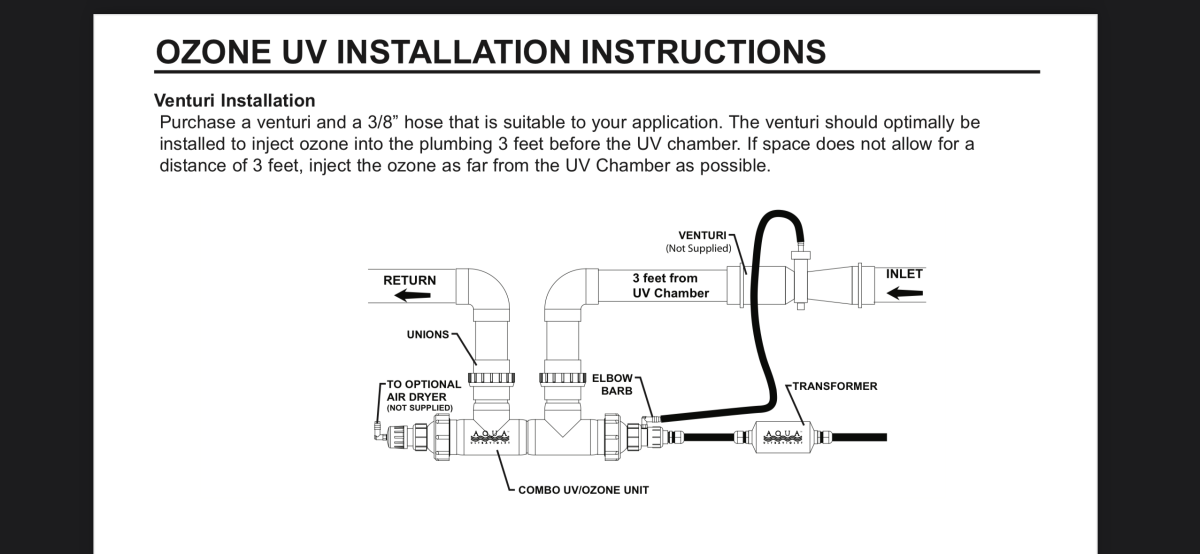
Illustration of combo I’m considering.



















