As you see in post #157, my Hanna checker does not see any nitrate for months now.That is exactly what happening with my experiment right now. I have almost zero (Salifert) Nitrate and Ammonia but .1-.2 ppm Nitrite for two weeks!
I was asking myself same questions why would this happen and why my nitrite-oxidizing bacteria (NOB) are not dealing with Nitrite?
a) lower affinity of nitrifying bacteria for nitrite - that is interesting but in NSW nitrite level is quite low often below .005 and still NOB are thriving.
b) competitive inhibition by chloride ions - I know this is valid for fish but never heard if valid for bacteria NOB will check
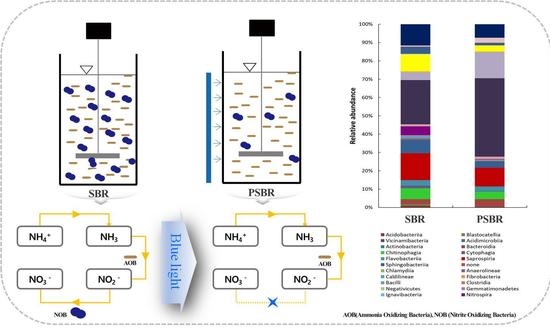
c) Inhibition by (blue) light - There is information that oxidation of nitrite is light inhibited especially by blue light
d) skimming - I was wondering since most of the NOB are inhabiting water column may be they are skimmed out and given their very slow reproduction rate might be a problem?
Would it be useful information for me to check for nitrite? It's been years since I have, lol.