- Joined
- Sep 20, 2013
- Messages
- 1,074
- Reaction score
- 1,152
Abstract: A calculation error led to the overdosing of tri sodium phosphate (TSP: Na3PO4) to a 700L (135 gallon display plumbed to two 25 gallon frag tanks) mixed reef system with deep sand bed that has been operating for the past 14 years. The overdose resulted in not the targeted 0.010 ppm Phosphate level, but 1000 times more. Instead of responding with the traditional course of successive water changes, increased application of ferric oxide (GFO), the system was allowed to operate as if nothing had happened. Bubble and hair algae growth did increase, but not disastrously. Coral (LPS, SPS) health improved. Phosphate levels returned to non-detect levels after 1 month.
System design
Initial water parameters:
Background
Effects of high phosphate

Current Practice
Current Water parameters
Learning points
I’m curious if anyone has any insights, theories or questions after reading this article. If you spot any flaws in my approach or analysis, please post a reply
System design
- Display tank: 135 gallon Oceanic glass
- Frag tanks: 2 x 25 gallon Tupperware tubs plumbed in series and back to system sump.
- Top-off: RO water passed through a calcium hydroxide reactor; effluent is <6ppb-PO4 .
- Lighting: 3x250W MH + 4 T5 over display; 1x250W MH + 2 T5 over both frag tanks
- Circulation: 2x Jebao RW-8 in display, 1x Jebao RW-8 in each frag tank
- Sump: DIY 20 gal. aquarium with Iwaki WMD30RLT return pump common to entire system.
- CO2 reactor: DIY
- Deep Sand bed (2-5”): in place for 14 years with periodic additions from beach vacations.
- DIY protein skimmer
- Minimal use of carbon (ROX 0.8)
Initial water parameters:
- Calcium: 390-440 ppm
- Alkalinity: 165-210 ppm CaCO3 (9.3 – 11.7 dKH)
- Mg: 1250-1350 ppm
- pH: 7.8 – 8.25
- Temperature: 77-79 oF
- Lights on: 8 hrs/day
Background
Display tank is modestly populated with soft corals (xenia, mushrooms), LPS (goniopora, wall hammer, torch coral), various SPS, 1 large blue clam. Two to three years ago, Phosphate levels were in the 10-30 ppb-PO4 range (Hanna ULR checker), nitrates were non-detect using a Seachem kit and using a Nylos kit. At that time, goniopora stokesi was reproducing, LPS population was expanding rapidly, SPS had minimally acceptable coloration and growth. There was very little hair algae and a small amount of bubble algae.
Two years ago, a power loss caused a system upset where all the fish perished, except 3 percula clown fish. The coral was not visibly affected. Shortly after that time, 80% of the LPS population was sold off for frags because they were beginning to overwhelm the system. At this point, the goniopora, SPS and remaining LPS began a slow decline. The reason for the decline was not known. In an attempt to improve things, the system was shifted to an ULNS (ultra low nutrient system) reaching non-detect levels of nitrate and phosphate using GFO and carbon dosing (first ethanol, then vinegar).
Two years ago, a power loss caused a system upset where all the fish perished, except 3 percula clown fish. The coral was not visibly affected. Shortly after that time, 80% of the LPS population was sold off for frags because they were beginning to overwhelm the system. At this point, the goniopora, SPS and remaining LPS began a slow decline. The reason for the decline was not known. In an attempt to improve things, the system was shifted to an ULNS (ultra low nutrient system) reaching non-detect levels of nitrate and phosphate using GFO and carbon dosing (first ethanol, then vinegar).
The ULNS had a few noticeable consequences:
Based on research and reflection, it was decided to begin nitrate dosing with calcium nitrate to maintain 1ppm-NO3 levels (Nyos test kit). Shortly after 2x daily nitrate additions with a dosing pump and maintaining 1ppm-NO3 levels, the SPS began to increase color. Torch corals still look pathetic. Phosphate levels remained at non-detect (Hanna ULR checker). Following the thought that adding nitrate was a good thing, it was decided to start dosing phosphate in order to maintain a level of 10 ppb-PO4.
- The need to clean aquarium glass dropped dramatically
- The film on the glass went from green or tan to milky white
- Bubble algae population declined, but did not go away.
- Hair algae population dropped.
- Chaetomorphia nearly completely perished.
- Steady and rapid LPS coral decline with hair algae growing near polyps.
- SPS population initially improved color vibrancy while growth rate improve marginally.
- SPS color eventually fell back to previous levels and growth rates stalled.
Based on research and reflection, it was decided to begin nitrate dosing with calcium nitrate to maintain 1ppm-NO3 levels (Nyos test kit). Shortly after 2x daily nitrate additions with a dosing pump and maintaining 1ppm-NO3 levels, the SPS began to increase color. Torch corals still look pathetic. Phosphate levels remained at non-detect (Hanna ULR checker). Following the thought that adding nitrate was a good thing, it was decided to start dosing phosphate in order to maintain a level of 10 ppb-PO4.
By confusing ppm and ppb, a calculation error led to the accidental overdose of TSP to the system. This happened on October 6, 2016. The amount dosed was 1000 times greater than intended and the system reached 10,000 ppb-PO4 instead of 10 ppb-PO4 [0.010 ppm] (Hanna ULR checker using sample dilution to bring into quantifiable range).
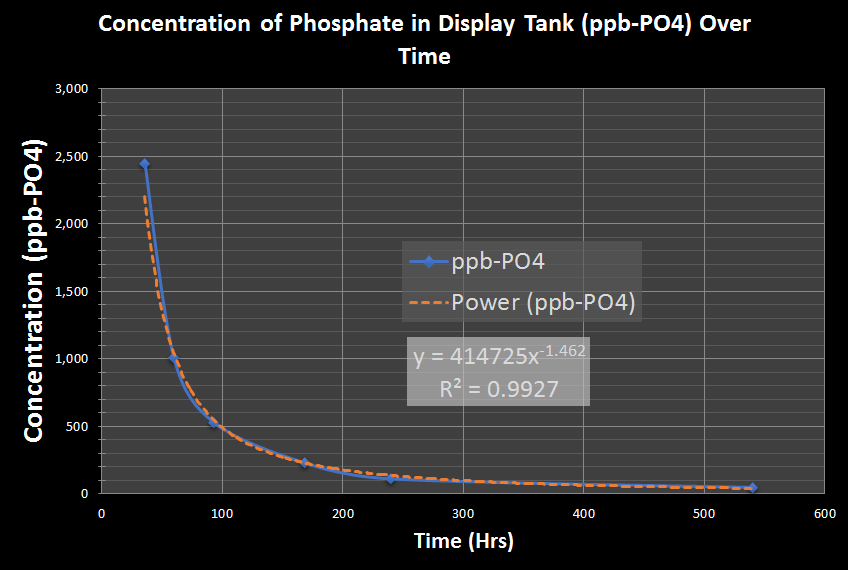
Instead of taking evasive action to immediately correct the problem, it was deiced to see what might happen if no corrective action was taken. Feeding routine was maintained and water changes were stopped to determine how long it would take the system to “consume’ the phosphate. Below is a graph showing the rate of phosphate depletion from the water column.
Instead of taking evasive action to immediately correct the problem, it was deiced to see what might happen if no corrective action was taken. Feeding routine was maintained and water changes were stopped to determine how long it would take the system to “consume’ the phosphate. Below is a graph showing the rate of phosphate depletion from the water column.
The graph ends after 22.5 days with a value of 49 ppb-PO4. The phosphate levels dropped to zero after another 5 more days (Novermber 3, 2016).
Effects of high phosphate
There was a noticeable increase in hair algae in the frag tanks but not at all in the display tank. Bubble algae broke out and covered areas of live rock in display tank and walls in the frag tank that was open to light and not heavily populated with other coral. The health of the goniopora (polyp extension, coloration) improved. Scaring of the tissue around the skeleton of a red goniopora has begun to heal. Coloration and growth of SPS improved.
The amount of bubble algae has begun to decline from its population maximum of a few weeks ago. It has not returned to nearly the low levels it was during the ULNS conditions. However, 5 emerald crabs were added. They are not seen eating bubble algae cells >2mm diameter, but they might be eating very small bubble algae cells <1mm.
Below are two pictures of the bubble algae at the worst of the outbreak and currently (November 19, 2016).
Below are two pictures of the bubble algae at the worst of the outbreak and currently (November 19, 2016).
Current Practice
The system is being dosed with calcium nitrate and TSP to maintain 1ppm-NO3 and 10 ppb-PO4. Heavy feeding is still employed. Both nitrate and phosphate are measured weekly to monitor accumulation of them. The system continues to consume nitrate and phosphate. It is not clear if these are being taken up by coral and algae, or processed in the deep sand bed and then removed via protein skimming. Large clumps of hair or bubble algae are removed when found, but this is not likely to explain the consumption of phosphate that is added by food and TSP additions.
Current Water parameters
- Calcium: 406 ppm
- Alkalinity: 183 ppm CaCO3 (10.2 dKH)
- Mg: 1270 ppm
- pH: 8.22 – 8.38
- Temperature: 77-79 oF
- Lights on: 8 hrs/day
Learning points
- For critical applications, second person verify calculations (use a spouse, reef mate or user groups).
- Making small changes to a system also means avoiding the removal of a large amount of one particular biomass (LPS) from the system all at one time.
- High phosphate levels are not necessarily going to lead to disaster [in this system].
- This system absorbed 12.8 grams of TSP in about 1 month.
- This system absorbed 12.8 grams of TSP in about 1 month.
- ULNS (non-detect levels of nitrate and phosphate) did not lead to healthy corals in this system.
- Having access to accurate water testing kits is essential.
- It is amazing how nature can restore and rebalance a biotope such as a reef aquarium after a momentary disturbance when one gives it a chance and does not interfere.
I’m curious if anyone has any insights, theories or questions after reading this article. If you spot any flaws in my approach or analysis, please post a reply