- Joined
- Sep 15, 2019
- Messages
- 20
- Reaction score
- 15
I never said anything about O2 not being needed. Not sure why you keep claiming otherwise.
I think, as Taricha mentions, you are incorrectly correlating high CO2 with low O2, and hence are misunderstanding my claims That is just not the case in a reef tank. CO2 and O2 are not opposites. Both can be high, both low, or one high and one low. To claim you want low CO2 because that means you have high O2 is just not correct.
For others who might be interested, let's explore that issue in detail.
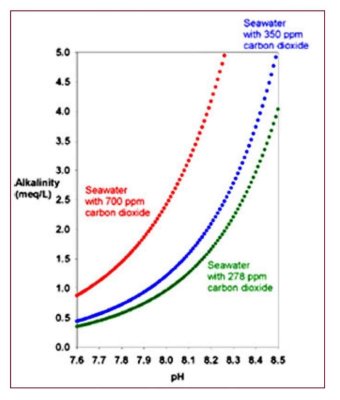
1. Folks who use high pH alkalinity additives such as sodium carbonate or calcium hydroxide (limewater/kalkwasser) reduce the CO2 in the tank, often to values well below that which is in equilibrium with normal air. But that process does absolutely nothing to raise (or lower) O2.
2. Folks who use CaCO2/CO2 reactors add CO2 to their aquaria, lowering pH and raising CO2 levels, often with CO2 ending up well above normal levels (pH below 8.1). Such reactors typically do nothing to raise or lower O2.
3. What about aeration with low vs high CO2 air? How does that impact O2? Let's see. Normal air is about 209,500 ppm O2 and about 400 ppm CO2. Normal saturation of seawater with O2 is about 6.6 mg/L at 25 deg C.
What if we have air where someone has breathed and converted some O2 to CO2, resulting in a 50% increase in CO2 to 600 ppm? How much does O2 drop? Since the total pressure hasn't changed (we made one CO2 from one O2), the O2 drops from 209,500 ppm to about 209,300 ppm. That changes the O2 pressure in the air by 0.095%.
How will that impact the O2 level in seawater that is equilibrated with that air? The solubility is directly related to the O2 pressure, so the 6.600 mg/L O2 in the seawater drops to 6.594 mg/L. That seems totally insignificant.
To compare that drop, it is much smaller than the change in O2 solubility in seawater from a 1 deg C change in the water temp. 25 deg C to 26 deg C causes a drop in O2 solubility from 6.600 mg/L to 6.500 mg/L.
What about pressure changes from barometer changes due to weather patterns? Those too are much bigger. A typical change in barometric readings is several whole number percent of the reading (much bigger in storms). A 2% drop in the barometric pressure will change the saturation level of O2 in seawater from 6.6 mg/L to below 6.5 mg/L. Much bigger change that that caused by in-home high CO2 air.
Thus, if high O2 is the goal during a treatment, you are far better off reducing the temperature and doing the work during a high pressure weather pattern than in worrying about the CO2 level in the air you are aerating with.
Would you please show me how high C02, and high oxygen can exist at the same time, with degassing?