Oxydators don't work super fast so be patient. If you want to get rid of the yellow quickly, add some carbon.1.5 days with Oxydator A with 2 cat and 12% peroxide. 90g tank. Water still yellow, no improvement at all.
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Anyone using Dr. Sochting's Oxydator
- Thread starter PapaDragon
- Start date
- Tagged users None
Sometimes they do in fact while sometimes it takes a good few days.Oxydators don't work super fast so be patient. If you want to get rid of the yellow quickly, add some carbon.
1.5 days is not enough - you may need a month or so before you see improvements, especially if you have refugium and the tank is mature.
Sincerely Lasse
Sincerely Lasse
- Joined
- Jul 26, 2018
- Messages
- 499
- Reaction score
- 213
It was full:How much peroxide has been used up?
Attachments
That doesn't look full to me. Your peroxide should last about a week give or take.It was full:
- Joined
- Dec 28, 2016
- Messages
- 22,830
- Reaction score
- 21,964
no clue what this means. and 1.5 days is a short period - but you just showed a green circle?1.5 days with Oxydator A with 2 cat and 12% peroxide. 90g tank. Water still yellow, no improvement at all.
- Joined
- Dec 28, 2016
- Messages
- 22,830
- Reaction score
- 21,964
First - what is that green circle??? that looks like you have a very dirty tank - or you're taking pictures of something not related? Maybe I'm stupid
- Joined
- Jul 26, 2018
- Messages
- 499
- Reaction score
- 213
It was full 1.5 days ago.That doesn't look full to me. Your peroxide should last about a week give or take.
It's water clarity test. White bucket will show if water is clear or not.First - what is that green circle??? that looks like you have a very dirty tank - or you're taking pictures of something not related? Maybe I'm stupid
1.5 days is not enough - you may need a month or so before you see improvements, especially if you have refugium and the tank is mature.
Sincerely Lasse
Laisse,
With respect to Oxydator, can you help me better understand how the process is metered?
I must ask this question. Would adding oxygen to intake of protein skimmer assist with oxidation potential of water? Is the net effect of adding peroxide, to increase oxidation potential of water?
I´ll try to explain the oxydator the way I understand it. Details can be wrong but I think I know the basic rather well
You fill up the plastic container with peroxid between 3 and 12 % strength In this case they use two catalysts - but all from 1 to whatever works.
 When you add the peroxide the process starts with help of the catalysts. H2O2 will be broken down to water and different form of oxygen radicals - in some cases depending on the catalyst. In this closed container - the result will be oxygen gas in the end. The gas will form a layer above the peroxide - as more gas will be produced - the pressure from the gas bubble in the top of the acrylic container will press non-catalyzed peroxide outside the acrylic container. (red lines). How fast the gas-bubble in the acrylic container will expand. hence press out more or lesser un catalyzed H2O2 outside the container depends on the H2O2 concentration and the amount of catalysts. Higher percent and more catalysts - more un catalysed H2O2 will be pressed out per time unit. This is valid for temperature too - higher temperature - faster reactions per time unit. In this case - the A-type - the un catalysed H2O2 will pas by the ceramic container (tonbecher). This serve as an catalysts too and if the rate of un catalysed H2O2 is low enough - all will be converted to oxygen gas. The oxydator serve as an source for oxygen in the aquarium.
When you add the peroxide the process starts with help of the catalysts. H2O2 will be broken down to water and different form of oxygen radicals - in some cases depending on the catalyst. In this closed container - the result will be oxygen gas in the end. The gas will form a layer above the peroxide - as more gas will be produced - the pressure from the gas bubble in the top of the acrylic container will press non-catalyzed peroxide outside the acrylic container. (red lines). How fast the gas-bubble in the acrylic container will expand. hence press out more or lesser un catalyzed H2O2 outside the container depends on the H2O2 concentration and the amount of catalysts. Higher percent and more catalysts - more un catalysed H2O2 will be pressed out per time unit. This is valid for temperature too - higher temperature - faster reactions per time unit. In this case - the A-type - the un catalysed H2O2 will pas by the ceramic container (tonbecher). This serve as an catalysts too and if the rate of un catalysed H2O2 is low enough - all will be converted to oxygen gas. The oxydator serve as an source for oxygen in the aquarium.
In my case - it is my reserve if I get a power breakdown. Normally it is in my sump but in case of power break down - I move it to the DT.
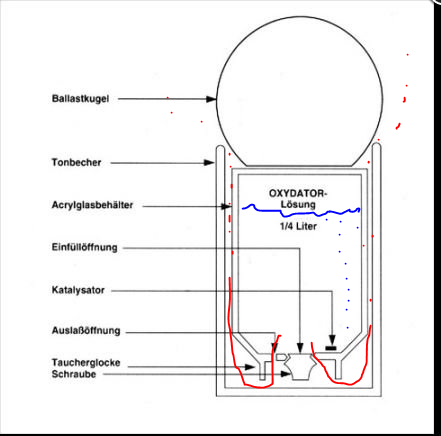
Of interest for me is was happen with the H2O2 that will past through the both "catalysts chambers" and end up as H2O2 in the water column. It will be breaken down with help of different compounds - iron is one. In this case oxygen radicals like single O atoms, HO radicals and HOO radicals will be formed. These radicals will oxidize whatever it encounter including organic matter. This is the way it works on yellow substances (read humic acids). But oxygen radicals does not differentiate between living and dead organic matter - it will oxidize the thing its first encounter. Therefore - the higher concentration of un catalyzed H2O2 that slips through the ceramic container - the higher is the statistic chance that it damage living organism behind their ability to repair. its a balance act. Can it be metered?
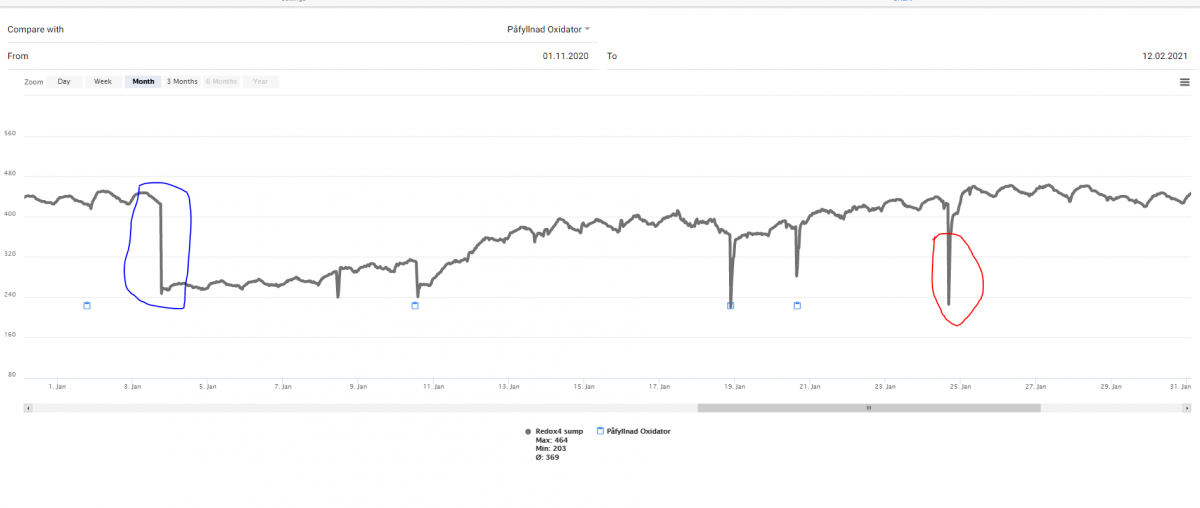
In comparison with the other method using oxygen radicals - ozone - the redox potential is tricky in this case. If you add pure H2O2 (or to much slips past the ceramic in the outer container) you will see a rather huge decline of redox potential - followed of a rise up to the level before and higher. The chart below is interesting in order to show this and that H2O2 can be mor persistent in our water compared with former thoughts (a matter of hours before total conversion). I have heard people working with peroxide as an antiworm agent in fish farms saying the same - it will exist longer than we think. My graph can give some suport for that claim
From the left - normal variation depending on pH. The fast decline around 2021-01-03 caused of an attempt of me to eradicate some aiptasia. I add around 30 ml 12% H2O2 in one of my BTN modules there one of my Wavemakers are hidden (yes I need no comments about that decision - even the sun have patches ) My tank is around 300 L (80 G).
) My tank is around 300 L (80 G).
However - it gave me a beautiful graph and maybe some more understandings of the mechanisms involved. H2O2 is a oxidizing agent - why will adding fresh H2O2 result in a dip of redox potential - it should be the opposite as with ozone. The small blue dots is marking for refilling of the oxydator - there will always be som spill when it restart. The red marked dip is not a refill but I took up the acrylic container and inspect it.
The breakdown of H2O2 is for me two different processes and this can explain these dips and the rise afterwards. By definition - when a compound lose a oxygen atom - it is a reduction and if it gain an oxygen atom - it is an oxidation.
By definition - when H2O2 lose one of its O atoms - a reduction process take place (lower the ORP). On the other hand when the radicals (O, HO and HOO) encounter something it can oxidize - an oxidation take place (higher the ORP). If your redox will decline or rise is therefore depended of which process dominate over the other. When just adde - much H2O2 lose one of its O atoms - a force taking ORP down exist and dominate - The radicals start to oxidize - a force taking ORP up exist and slower or dominate over the reducing process according to ORP. When pure H2O2 in the water column get lower and lower - ORP stabilize and rise to the old level (or slightly higher)
I´m total aware that it could be done thousands of studies in detail but this is my explanation in a macro plane - or could be an explanation.
If I´m right - some H2O2 may have a lifetime around 20 days in an aquarium. I´m aware of that this is one test - I should redo it in order to validate the results - but after the first addition of more than 30 ml 12% H2O2 - my hearth attack was not long away - nearly all of my corals reacts with shrinking and some have not totally recover yet. Fish and other animals - no reactions.
If it should be used - as ozone - together with a skimmer - I do not know. For the moment - I have my oxydator in the same apartment as my skimmer - some will come into the skimmer - bad or good - i do not really know - but it should not been my first choose if I had a possibility to place it on another place.
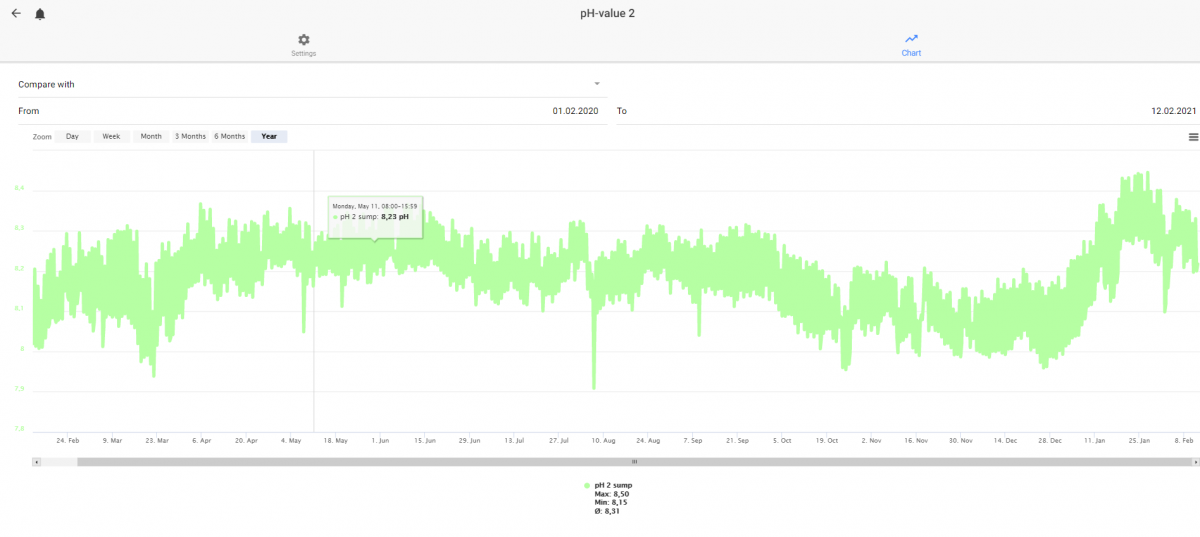
I have also noted a funny reaction from my pH probe when I move the oxydator to the same part in the sump as I have my probes. My pH shows summer behavior - even if it is a cold winter with very closed windows and doors. High CO2 indoors.
My pH the last year. No other changes and the CO2 levels in the room with my aquarium have warned us about CO2 above 1000 ppm the last days. I moved the Oxydator 2021-01-10. The pH probe is calibrated around 1 of february.
I hope that I at least answered some of your questions.
Sincerely Lasse
You fill up the plastic container with peroxid between 3 and 12 % strength In this case they use two catalysts - but all from 1 to whatever works.
In my case - it is my reserve if I get a power breakdown. Normally it is in my sump but in case of power break down - I move it to the DT.
In comparison with the other method using oxygen radicals - ozone - the redox potential is tricky in this case. If you add pure H2O2 (or to much slips past the ceramic in the outer container) you will see a rather huge decline of redox potential - followed of a rise up to the level before and higher. The chart below is interesting in order to show this and that H2O2 can be mor persistent in our water compared with former thoughts (a matter of hours before total conversion). I have heard people working with peroxide as an antiworm agent in fish farms saying the same - it will exist longer than we think. My graph can give some suport for that claim
From the left - normal variation depending on pH. The fast decline around 2021-01-03 caused of an attempt of me to eradicate some aiptasia. I add around 30 ml 12% H2O2 in one of my BTN modules there one of my Wavemakers are hidden (yes I need no comments about that decision - even the sun have patches
However - it gave me a beautiful graph and maybe some more understandings of the mechanisms involved. H2O2 is a oxidizing agent - why will adding fresh H2O2 result in a dip of redox potential - it should be the opposite as with ozone. The small blue dots is marking for refilling of the oxydator - there will always be som spill when it restart. The red marked dip is not a refill but I took up the acrylic container and inspect it.
The breakdown of H2O2 is for me two different processes and this can explain these dips and the rise afterwards. By definition - when a compound lose a oxygen atom - it is a reduction and if it gain an oxygen atom - it is an oxidation.
By definition - when H2O2 lose one of its O atoms - a reduction process take place (lower the ORP). On the other hand when the radicals (O, HO and HOO) encounter something it can oxidize - an oxidation take place (higher the ORP). If your redox will decline or rise is therefore depended of which process dominate over the other. When just adde - much H2O2 lose one of its O atoms - a force taking ORP down exist and dominate - The radicals start to oxidize - a force taking ORP up exist and slower or dominate over the reducing process according to ORP. When pure H2O2 in the water column get lower and lower - ORP stabilize and rise to the old level (or slightly higher)
I´m total aware that it could be done thousands of studies in detail but this is my explanation in a macro plane - or could be an explanation.
If I´m right - some H2O2 may have a lifetime around 20 days in an aquarium. I´m aware of that this is one test - I should redo it in order to validate the results - but after the first addition of more than 30 ml 12% H2O2 - my hearth attack was not long away - nearly all of my corals reacts with shrinking and some have not totally recover yet. Fish and other animals - no reactions.
If it should be used - as ozone - together with a skimmer - I do not know. For the moment - I have my oxydator in the same apartment as my skimmer - some will come into the skimmer - bad or good - i do not really know - but it should not been my first choose if I had a possibility to place it on another place.
I have also noted a funny reaction from my pH probe when I move the oxydator to the same part in the sump as I have my probes. My pH shows summer behavior - even if it is a cold winter with very closed windows and doors. High CO2 indoors.
My pH the last year. No other changes and the CO2 levels in the room with my aquarium have warned us about CO2 above 1000 ppm the last days. I moved the Oxydator 2021-01-10. The pH probe is calibrated around 1 of february.
I hope that I at least answered some of your questions.
Sincerely Lasse
Last edited:
I do not think my NO3 get lower from the Oxydator, but from the siporax and more water changes. Clarity of water and cleaner Glasses and cleaner look is the reason i continue use Oxydator. I dont use activated carbon at all to my tank.Nice tank! How/why does the oxidator have an impact on your nitrates?
Nice tank! How/why does the oxidator have an impact on your nitrates?
Nice tank! How/why does the oxidator have an impact on your nitrates?
Hydrogen peroxide reacts with ammonia probably converting it to nitrate perhaps by first converting it to nitrite but am not sure of the actual reaction. However, I can tell you peroxide does nullify ammonia probably by oxidizing it. Perhaps @Lasse can explain the reason and chemical reaction better.
NH3/NH4 -> NO2 -> NO3. This is a biological oxidation done by microorganisms like archaea and bacteria.
I know that many people have suggest that adding H2O2 will chemical do t5he same thing. However - I have never seen any reports or studies that support a chemical oxidation of NH4 to NO2 by H2O2. There is studies that support the ideas that oxygen gas created by the breakdown of H2O2 have impact the biological nitrification rate - but it still the microorganisms that do the real oxidation. IMO - if a chemical oxidation should take place - it needs much, much more higher H2O2 concentration compared with these we are working with @Randy Holmes-Farley may have more information about this
Next question - could nitrite (NO2) be converted into Nitrate (NO3) by chemical H2O2 oxidation? As I know it for the moment - no but indirect by higher oxygen gas content. However - IMO - if there is any oxidation by H2O2 of inorganic nitrogen species - oxidation of NO2 is more likely than others. A combination with UV can change this IMO.
Sincerely Lasse
I know that many people have suggest that adding H2O2 will chemical do t5he same thing. However - I have never seen any reports or studies that support a chemical oxidation of NH4 to NO2 by H2O2. There is studies that support the ideas that oxygen gas created by the breakdown of H2O2 have impact the biological nitrification rate - but it still the microorganisms that do the real oxidation. IMO - if a chemical oxidation should take place - it needs much, much more higher H2O2 concentration compared with these we are working with @Randy Holmes-Farley may have more information about this
Next question - could nitrite (NO2) be converted into Nitrate (NO3) by chemical H2O2 oxidation? As I know it for the moment - no but indirect by higher oxygen gas content. However - IMO - if there is any oxidation by H2O2 of inorganic nitrogen species - oxidation of NO2 is more likely than others. A combination with UV can change this IMO.
Sincerely Lasse
Thanks Lasse,NH3/NH4 -> NO2 -> NO3. This is a biological oxidation done by microorganisms like archaea and bacteria.
I know that many people have suggest that adding H2O2 will chemical do t5he same thing. However - I have never seen any reports or studies that support a chemical oxidation of NH4 to NO2 by H2O2. There is studies that support the ideas that oxygen gas created by the breakdown of H2O2 have impact the biological nitrification rate - but it still the microorganisms that do the real oxidation. IMO - if a chemical oxidation should take place - it needs much, much more higher H2O2 concentration compared with these we are working with @Randy Holmes-Farley may have more information about this
Next question - could nitrite (NO2) be converted into Nitrate (NO3) by chemical H2O2 oxidation? As I know it for the moment - no but indirect by higher oxygen gas content. However - IMO - if there is any oxidation by H2O2 of inorganic nitrogen species - oxidation of NO2 is more likely than others. A combination with UV can change this IMO.
Sincerely Lasse
I had a cousin phone me a while ago as all his tropical fish were dying. He had far to many fish for his size of tank, where have we read that before. Anyway, I told him to dose x amount of 3% peroxide every 15 mins untill he could see bubbles on the plants or sides of the tank. This he did and all fish recovered he never lost one. I liken this to putting an oxygen mask on his fish but in some way it appears to have reduced/ prevented ammonia burning the fishes gills which are sensitive to ammonia poisoning or burning. I have done this myself or advised people a few times over the years with fish suffering similar with the same success.
Was it freshwater fish and do you know the pH?Thanks Lasse,
I had a cousin phone me a while ago as all his tropical fish were dying. He had far to many fish for his size of tank, where have we read that before. Anyway, I told him to dose x amount of 3% peroxide every 15 mins untill he could see bubbles on the plants or sides of the tank. This he did and all fish recovered he never lost one. I liken this to putting an oxygen mask on his fish but in some way it appears to have reduced/ prevented ammonia burning the fishes gills which are sensitive to ammonia poisoning or burning. I have done this myself or advised people a few times over the years with fish suffering similar with the same success.
Sincerely Lasse
Sorry I should have said. Freshwater fish and unfortunately he didn't know the PH of the tank water. He lives a long way from me so I couldn't just pop over myself.Was it freshwater fish and do you know the pH?
Sincerely Lasse
Hiwever,I did save a friends tang in a similar way
With the freshwater fish - I could be either oxygen diefency or nitrite poising. The nitrite poising cause that some of the blood cells can´t take up oxygen as they use to do. Given more oxygen into the water help the fish to get oxygen in spite that the uptake rate is lesser. The higher oxygen pressure will also give the nitrification cycle a boost.
With the tang - you can ignore the nitrite - the uptake into the fish bloodstream is blocked by chloride ions, However - ammonia can "burn" the gills and a higher oxygen level caused by the H2O2 help the fish to survive.
Sincerely Lasse
With the tang - you can ignore the nitrite - the uptake into the fish bloodstream is blocked by chloride ions, However - ammonia can "burn" the gills and a higher oxygen level caused by the H2O2 help the fish to survive.
Sincerely Lasse
Thanks Lasse,With the freshwater fish - I could be either oxygen diefency or nitrite poising. The nitrite poising cause that some of the blood cells can´t take up oxygen as they use to do. Given more oxygen into the water help the fish to get oxygen in spite that the uptake rate is lesser. The higher oxygen pressure will also give the nitrification cycle a boost.
With the tang - you can ignore the nitrite - the uptake into the fish bloodstream is blocked by chloride ions, However - ammonia can "burn" the gills and a higher oxygen level caused by the H2O2 help the fish to survive.
Sincerely Lasse
I also think peroxide aids fight stress of newly introduced fish that show gaping and rapid mouth and gill. movement. Tangs in particular can be stressed so after first introduction esp following a long journey and cold water conditions with little oxygen in the bag.
Last edited:
So basically, its a slow dose of h202 depending on how many catalystis are producing gas to push out the h202 from the bottom. Then the h202 is broken down in the water column.
Similar threads
- Replies
- 1
- Views
- 168
- Replies
- 12
- Views
- 275
- Replies
- 1
- Views
- 217
- Replies
- 4
- Views
- 615


















