PeterC99
Solarbenchmark.com
View Badges
Excellence Award
Reef Tank 365
NJRC Member
Hudson Valley Reef Keepers
Hospitality Award
My Tank Thread
My Aquarium Showcase
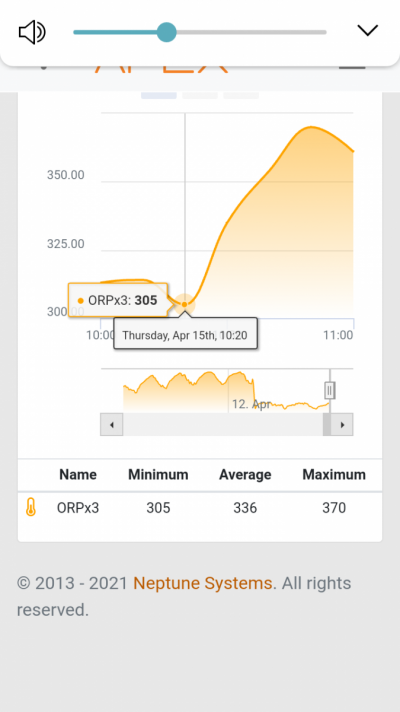
Just got my first Oxydator A from The Shrimp Tank! Setup with diluted 6% hydrogen peroxide and one catalyst to get started.
After 4 days, noticing my cyano receding. Wondering when I can use stronger strength HP and another catalyst?
After 4 days, noticing my cyano receding. Wondering when I can use stronger strength HP and another catalyst?