I agree that all my precip issues went away with dosing just plain baking soda as opposed to soda ash, I also have not noticed much of a PH decrease.
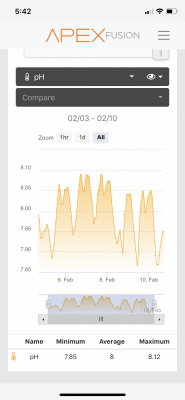
My PH ran at 8.5 when I was dosing soda ash, now its around 8.2 dosing bicarb. I don't have a white crust on my heaters or pumps either.
It's also easier to just pour more into RO/DI, then baking it, then adding.
My PH ran at 8.5 when I was dosing soda ash, now its around 8.2 dosing bicarb. I don't have a white crust on my heaters or pumps either.
It's also easier to just pour more into RO/DI, then baking it, then adding.