Now that you mention it , yes . Prior to TDS starting to show higher I was producing water in shorter time frame. ?Have you noticed if you are making water significantly faster than the rating of the membrane?
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Eating Up RO Membranes
- Thread starter flyfisher2
- Start date
- Tagged users None
Let me clarify, shorter in comparison to when I initially changed the membrane three months ago.Now that you mention it , yes . Prior to TDS starting to show higher I was producing water in shorter time frame. ?
Thank you for all the info and pointing out the potential risk with chloramines. Let me say that upon calling my water supply I was given the number to the tech at the plant with whom I discussed my issue and he reassured me that the only purifier used is in fact chlorine and not chloramine.
I've already begun the disassembly of the old system and I'm sanitizing and rebuilding it. Just waiting for the new unit with the membrane so I can get this up and running. I'm really feeling that the heat is a major culprit.
I see that you're in Florida as well. Are you having any problems with your RO/DI? How much water do you produce? What kind of life are you seeing with your filters? Lakeland is not far from me. I'm Lake County
Mine is inside and I am also on a well so I don‘t have the same issues. I think you’re probably right about the heat. I suspect the sun on the membrane housing when you aren’t using it is just heating up the water and the membrane to temperatures it doesn’t like, along with the fact that ambient air temperatures down here are oppressive at best during the summer LOL.
I make a bunch of water and my well water is pretty high TDS and I generally replace my membranes every 12-18 months. When they’re rejection rate starts to decline is when I put fresh ones in. I have a triple inline TDS meter so I have a sensor after the RO membrane, then one after a DI stage and the third one after the second DI stage. I can monitor the product water after the RO membrane to check it’s health, the one after the first DI stage is so I can see when TDS starts creeping through the first DI stage and I can rotate the second stage DI into the first stage, then add a fresh one to the second chamber.
I can vouch for the Spectrapure SpectraSelect membranes performing better than the average Dow Filmtech Membranes as well. I get a significantly better rejection rate with my high TDS water with them than the less expensive Dow ones, so there is something different with them. (Might be worth waiting to buy those until after you figure out if you’ve solved your premature membrane death issue). You also may not need that extra bit like my water supply does.
How cold is your cold water out of the tap?
the line sitting in the sun outside the house could certainly get over 110 deg. Depending on how long the line is in the sun, it’s possible that the temp is still high when it reaches the RO membrane, although I think it’s unlikely given the small amount of water in the line relative the large volume of water in the canisters before the RO.
But if the water is well over 77 deg for many gallons, then you certainly could be getting a much lower rejection rate.
do you have a pressure gauge on the system? If you told us your water pressure and cold water temp, it would help a ton.
Have you measured the waste water to RO water ratio? You should probably be at 3:1.
the line sitting in the sun outside the house could certainly get over 110 deg. Depending on how long the line is in the sun, it’s possible that the temp is still high when it reaches the RO membrane, although I think it’s unlikely given the small amount of water in the line relative the large volume of water in the canisters before the RO.
But if the water is well over 77 deg for many gallons, then you certainly could be getting a much lower rejection rate.
do you have a pressure gauge on the system? If you told us your water pressure and cold water temp, it would help a ton.
Have you measured the waste water to RO water ratio? You should probably be at 3:1.
my water is also relatively low TDS but high very high sediment. The area is relatively new and lots of construction so I see lots of sand in the pre filters.I personally use the little test strips for chlorine. Any hint of color and it gets changed. The big blue carbon is the way to go IMO. If you have the space. Much longer contact time so it lasts MUCH longer. I was burning through regular carbons. Now I’m 10k+ through the big blue and still going.
the other thing you need to keep in mind is water pressure. Need a minimum of 40psi at the RO membrane. The lower the pressure the less effective the membrane operates. I have a booster and keep my pressure at the membrane right at 80psi.
the more canisters and filters you put BEFORE the membrane the lower your pressure is going to be at the membrane.
i put a pressure gauge on each side of my sediment filter. When I see a 10% drop in pressure, I change the sediment.
you really only need a single .5 micron sediment then one ore two carbon then your RO then a couple DI.
I have .5 sediment -> big blue -> 2x 90gpd RO -> high capacity DI -> silica buster DI.
I have low TDS source water but high sediment. And also my water has the dreaded chloramine
You’ll see I have two empty canisters. These were previously my sediment and carbon. But empty now because of big blue.



Yes I shared the pressure in the early part of the post, the pressure at the gauge was reading at about 60 and the water temp at 8:30 AM was at 81F. The unit was not in direct sunlight and the feed hose neither but ambient temp in Florida hits 90's daily and the concrete does get hot. As it stands the unit is coming indoors so this will no longer be an issue. Just want to make sure that will solve the problem.How cold is your cold water out of the tap?
the line sitting in the sun outside the house could certainly get over 110 deg. Depending on how long the line is in the sun, it’s possible that the temp is still high when it reaches the RO membrane, although I think it’s unlikely given the small amount of water in the line relative the large volume of water in the canisters before the RO.
But if the water is well over 77 deg for many gallons, then you certainly could be getting a much lower rejection rate.
do you have a pressure gauge on the system? If you told us your water pressure and cold water temp, it would help a ton.
Have you measured the waste water to RO water ratio? You should probably be at 3:1.
I'll check the tap water temp....
Tap water temp at 7:30 AMYes I shared the pressure in the early part of the post, the pressure at the gauge was reading at about 60 and the water temp at 8:30 AM was at 81F. The unit was not in direct sunlight and the feed hose neither but ambient temp in Florida hits 90's daily and the concrete does get hot. As it stands the unit is coming indoors so this will no longer be an issue. Just want to make sure that will solve the problem.
I'll check the tap water temp....
Attachments
Brew12
Electrical Gru
View BadgesExcellence Award
Reef Tank 365
Article Contributor
Moderator Emeritus
North Alabama Reef Club
Article Administrator
My Tank Thread
Just as an FYI, calling the utility is the best way to find out if you have chloramines. However, chloramines break down into ammonia over time. If you test some older RODI and get ammonia, then chloramines are a likely source.I’d find a way to test for chloramines.
Far from foolproof but it's the best way to test that I know of.
Thanks for that info Brew, so just to be clear... "test older RO/Di" . You mean my ATO water in the Brute and timeframe? I can do that shortly.Just as an FYI, calling the utility is the best way to find out if you have chloramines. However, chloramines break down into ammonia over time. If you test some older RODI and get ammonia, then chloramines are a likely source.
Far from foolproof but it's the best way to test that I know of.
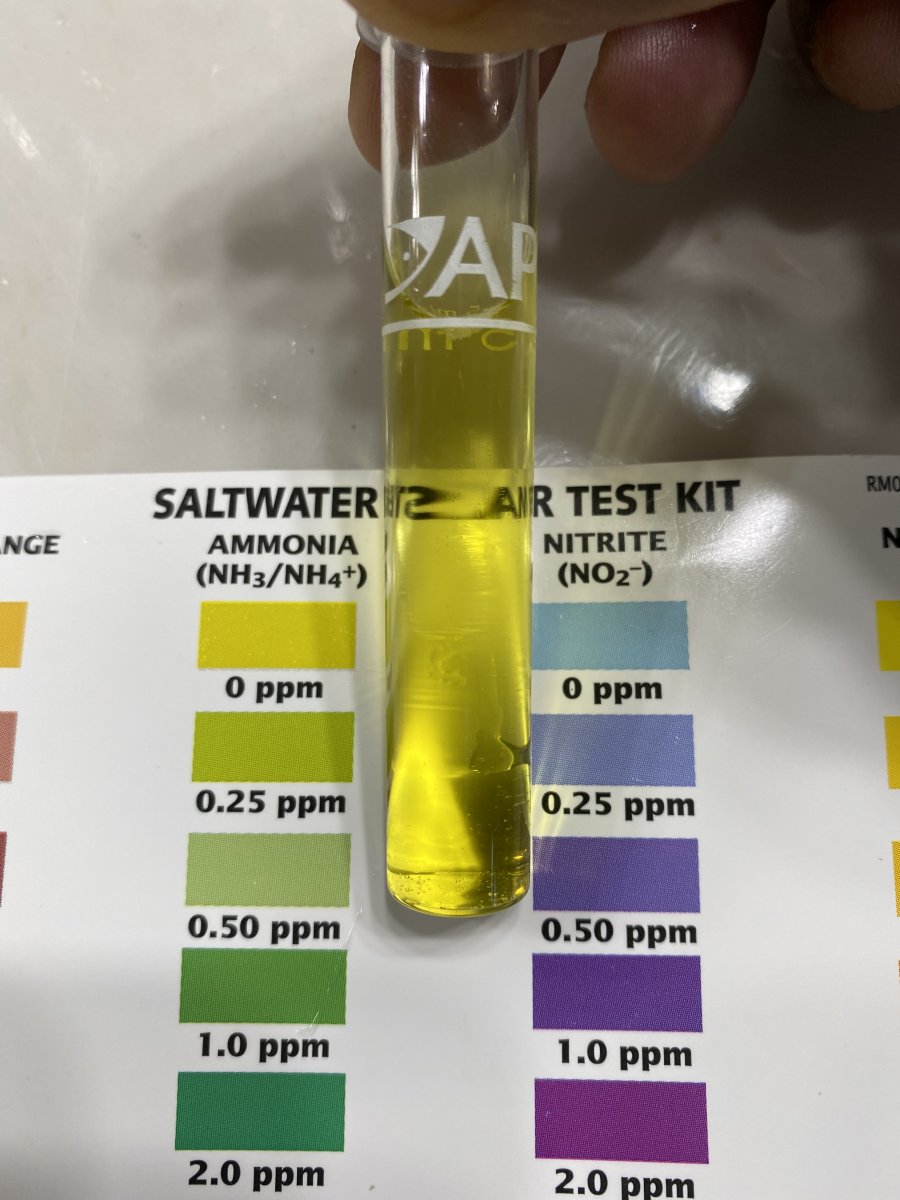
API is all I have available at the moment. The water is about 5 days old
Brew12
Electrical Gru
View BadgesExcellence Award
Reef Tank 365
Article Contributor
Moderator Emeritus
North Alabama Reef Club
Article Administrator
My Tank Thread
Yup, that should be old enough to begin showing, although I wasn't necessarily recommend that you test unless you didn't trust your water utility when they said they didn't use it.API is all I have available at the moment. The water is about 5 days old
I still suspect your issue is overheating while not in use from sunshine and low air flow to cool it. I would be surprised if moving it inside doesn't fix it.
Thanks, I just needed to be sure and have that added confidence. I do hope this fixes it and based on the info that you and others have shared, it would seem that heat is the number one culprit at this point.Yup, that should be old enough to begin showing, although I wasn't necessarily recommend that you test unless you didn't trust your water utility when they said they didn't use it.
I still suspect your issue is overheating while not in use from sunshine and low air flow to cool it. I would be surprised if moving it inside doesn't fix it.
That temp is just fine.Tap water temp at 7:30 AM
the big question will be what is your tap water temp mid or late day now.
once you’ve run the unit for a minute, the hot water from the sun is quickly replaced by the cooler water from the underground pipes.
pressure, temp and membrane damage are the only variables.(or water passing around an improperly seated membrane or leaking O ring)
given the normal amount of chlorine, and your changing of carbon, I highly doubt you’ve been damaging the membranes. So that leaves Temp.
those film Tec are rated at 50psi and 77 deg with a minimum rejection of 96%. So at that level you could be getting 6ppm passing through to your DI. Up your water temp and that goes up.
so if you tap water hits the 80s mid day, that’s probably the issue.
and there was no need to have replaced the membranes that quickly as they were functioning correctly. :/ just not handling the TDS well enough under your application conditions.
hopefully bringing everything inside lowers the temp enough to make a difference.
you should make it a practice to only make water first thing in the morning when the main line water is at its coolest.
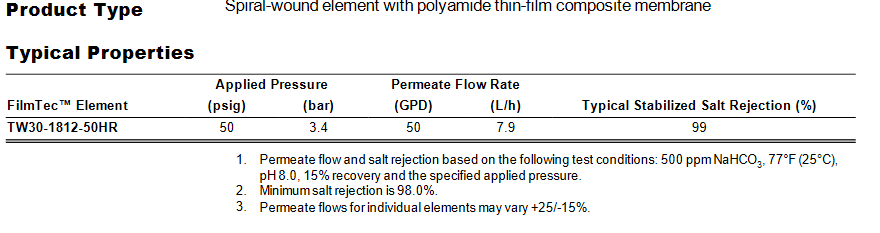
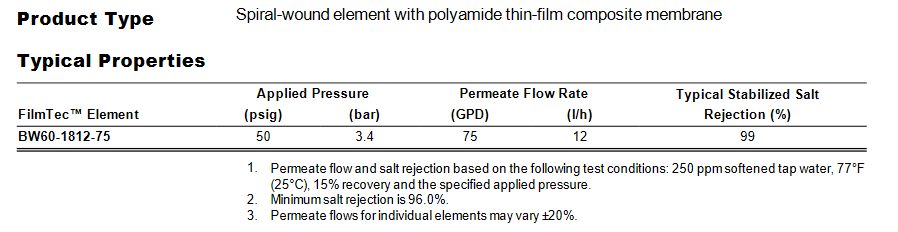
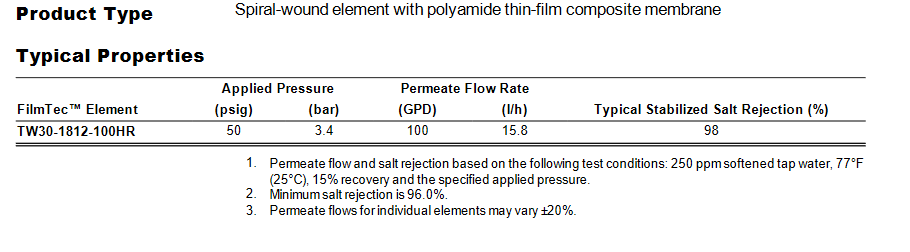
I was looking at specs for membranes and it looks like Dupont has updated some this year and used different specs for the testing. The 50 gpd membrane is now being tested at 500 ppm of sodium bicarbonate instead of 250 ppm softened tap water and has a min salt rejection of 98% instead of the others at 96%.
I do find it interesting that no one ever seems to mention the 15% recovery rate (5.7:1) that Dupont test the membranes at.
If you are really bored and want to read more about membranes this is the technical manual
I do find it interesting that no one ever seems to mention the 15% recovery rate (5.7:1) that Dupont test the membranes at.
If you are really bored and want to read more about membranes this is the technical manual
I was looking at specs for membranes and it looks like Dupont has updated some this year and used different specs for the testing. The 50 gpd membrane is now being tested at 500 ppm of sodium bicarbonate instead of 250 ppm softened tap water and has a min salt rejection of 98% instead of the others at 96%.



I do find it interesting that no one ever seems to mention the 15% recovery rate (5.7:1) that Dupont test the membranes at.
If you are really bored and want to read more about membranes this is the technical manual
the recovery rate is one part I’m still a little confused with re rejection rate. 5.7:1 is a ton of wasted water!
I have the 1:1 maxcap.
If they are getting 98% at 5.7:1 does that mean If you ran it at 1:1 you’d get a much lower rejection rate?
tagging along and listening guys. This is a learning experience.the recovery rate is one part I’m still a little confused with re rejection rate. 5.7:1 is a ton of wasted water!
I have the 1:1 maxcap.
If they are getting 98% at 5.7:1 does that mean If you ran it at 1:1 you’d get a much lower rejection rate?
I'll get you a schematic together tomorrow, and a parts list for your replacement filters, do with it as you wish, you won't hurt my feelings by not using everything I recommend. There are so many combinations you can setup and many different brands of filters.
One thing I would say though for sure is get a reliable and accurate chlorine test kit, and use that to judge when to replace your carbon, not an arbitrary time frame. Then you will know for sure you aren't hitting your RO membranes with chlorine.
Schematics below.
GOLDEN! That'll give you optimal RO production. It'll be the change in temp throughout the day that you'll have to keep an eye on.Tap water temp at 7:30 AM
the recovery rate is one part I’m still a little confused with re rejection rate. 5.7:1 is a ton of wasted water!
I have the 1:1 maxcap.
If they are getting 98% at 5.7:1 does that mean If you ran it at 1:1 you’d get a much lower rejection rate?
It would all depend on your water source. I think it would more reduce the life of the membrane and just a slight reduction in rejection rate. Hard water would be hardest on the membrane since those ions are also the most insoluble and could form scale if they reached the solubility point at the membrane.
@flyfisher2 Did not mean to derail your thread but it was already 14 pages.
my water is also relatively low TDS but high very high sediment. The area is relatively new and lots of construction so I see lots of sand in the pre filters.
If you are getting that much sediment i would recommend a whole house sediment filter.
Similar threads
- Replies
- 7
- Views
- 362
- Replies
- 1
- Views
- 100
- Replies
- 25
- Views
- 634
New Posts
-
Hello New here SoCal 180 gal Waterbox reef set up in the Man cave
- Latest: AlyciaMarie
-
Idaho Washington reefbreeder 48" photon v2 pro
- Latest: Fishy_mcfish



















