Were you sending only room/outside air through it or were you recirculating air through the skimmer?I’ve added a CO2 scrubber. I do not think it’s enough air draw to effectively scrub the air.
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
If its not CO2, then why low pH
- Thread starter Biologic
- Start date
- Tagged users None
Were you sending only room/outside air through it or were you recirculating air through the skimmer?
Fortunately the skimmer is smaller and uses 1/4 inch line so RO line works perfectly. I can have it recirculate or pull air from outside and then recirculate.
One thing that I find when pure recirculating mode, is that when it’s only recirculating the air in the system when the outside air shuts off, there is a vacuum in the system and the water gradually rises and makes the skimmer skim wet.
I’ve seen more positive affects on pH recirculate air being pulled from outside, than exclusively recirculating scrubbed air.
I can turn valves to change how the system pulls air.
Attachments
Interesting setup. I wonder what would happen if you closed in the top of the tank from the room? Not sure if that would cause an issue with O2 levels, but might not with gas exchange from the skimmer. Would stop the tank from taking in room CO2!Fortunately the skimmer is smaller and uses 1/4 inch line so RO line works perfectly. I can have it recirculate or pull air from outside and then recirculate.
One thing that I find when pure recirculating mode, is that when it’s only recirculating the air in the system when the outside air shuts off, there is a vacuum in the system and the water gradually rises and makes the skimmer skim wet.
I’ve seen more positive affects on pH recirculate air being pulled from outside, than exclusively recirculating scrubbed air.
I can turn valves to change how the system pulls air.
I have been tinkering with my tank to bump pH to stay within a certain range. Refugium counter cycle to the tank and lately dosing Kalk based on tank pH.
Seeing as how the outdoor air test worked.. if you don't wanna get a new skimmer maybe reduce the surface agitation caused by your wave maker pumps. This way most agitation will be from the skimmer with the outdoor airline and not be canceled out from the indoor air agitation.
Also the 1/4 RO line seems pretty small mine is quite a bit larger then that for the outdoor air. You didn't mention (or I missed it) how long the line is to outside from the skimmer?
Also the 1/4 RO line seems pretty small mine is quite a bit larger then that for the outdoor air. You didn't mention (or I missed it) how long the line is to outside from the skimmer?
Be aware of the fact that if you use a recirkulation method with a CO2 scrubber - you will not get any oxygen into the system either. It is the same air all the time but rinsed of CO2
Sincerely Lasse
Sincerely Lasse
Last edited:
Interesting setup. I wonder what would happen if you closed in the top of the tank from the room? Not sure if that would cause an issue with O2 levels, but might not with gas exchange from the skimmer. Would stop the tank from taking in room CO2!
I have been tinkering with my tank to bump pH to stay within a certain range. Refugium counter cycle to the tank and lately dosing Kalk based on tank pH.
I am actually going to cover my tank with clear plastic wrap and see what happens. It’s a Nano, so it’s not much trouble.
Seeing as how the outdoor air test worked.. if you don't wanna get a new skimmer maybe reduce the surface agitation caused by your wave maker pumps. This way most agitation will be from the skimmer with the outdoor airline and not be canceled out from the indoor air agitation.
Also the 1/4 RO line seems pretty small mine is quite a bit larger then that for the outdoor air. You didn't mention (or I missed it) how long the line is to outside from the skimmer?
I am going to see if I can avoid agitation but keep my sine wave working from my wave pumps.
the line is about 10 feet. I tested the output of the effluent volume flow before and after and there’s no difference in the water volume that the skimmer pump moves through the skimmer body. In fact, deletec suggests to throttle the air skimmer until you hear the pump make noise, then open the air valve (that little gray valve outside of my house) until pump makes no more noise. I am running wide open.
Be aware of the fact that if you use a recirkulation method with a CO2 scrubber - you will not get any oxygen into the system either. It is the same air all the time but rinsed of CO2
Sincerely Lasse
I agree. I don’t think recirculating CO2 scrubbers are long term impactful and beneficial enough to boost pH.
kalkwasser is infinitely better and can be controlled.
I am doing all this to prove that CO2 is my problem. But I am still not 100% convinced. Winter without Kalkwasser had similar numbers though. I need to get my indoor/outdoor CO2 meter to see what’s going on.
Interesting setup. I wonder what would happen if you closed in the top of the tank from the room? Not sure if that would cause an issue with O2 levels, but might not with gas exchange from the skimmer. Would stop the tank from taking in room CO2!
I have been tinkering with my tank to bump pH to stay within a certain range. Refugium counter cycle to the tank and lately dosing Kalk based on tank pH.
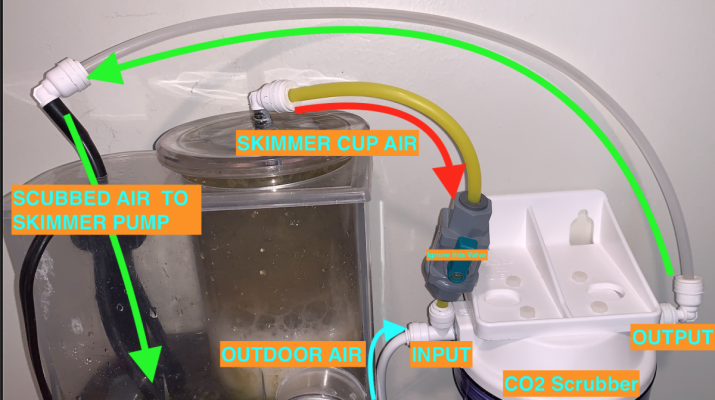
Incase the last CO2 Scrubber diagram was confusing. I cleaned the air plumping up and remade the diagram on the computer.
The valve is only there if I wish not to rescrub the air, and I'd just take the skimmer cup air tube out completely.
Attachments
We are two person living in our flat and CO2 vary a lot. When our grandchildren visit us - we could see on my aquarium pH when they wake up in the morning before I start to dose Core 7 3 a+b mostly in the night and use outside air. Core 7:3a+b is Na2CO3 and ir rise pH.
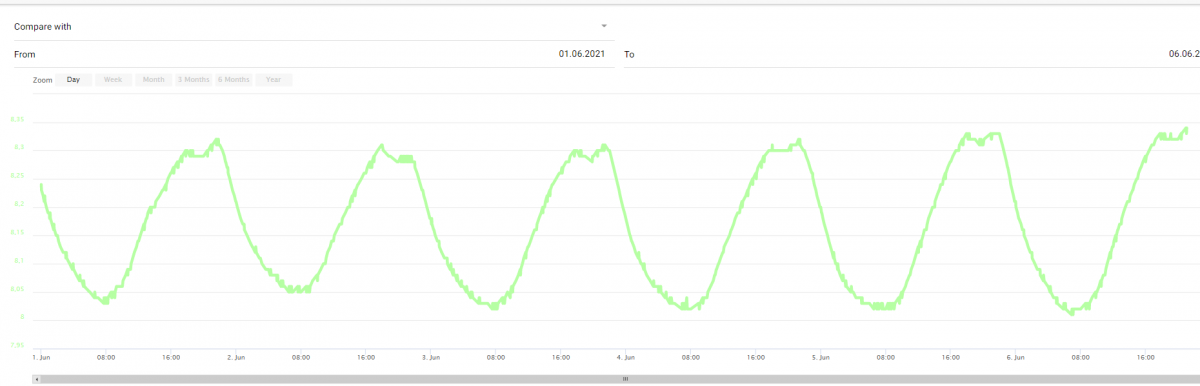
pH last week
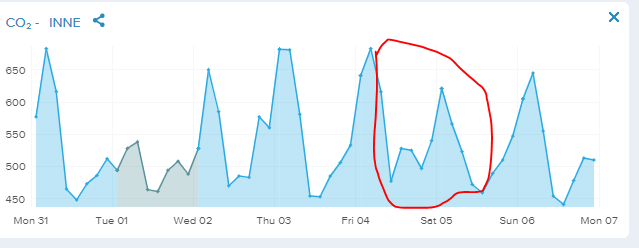
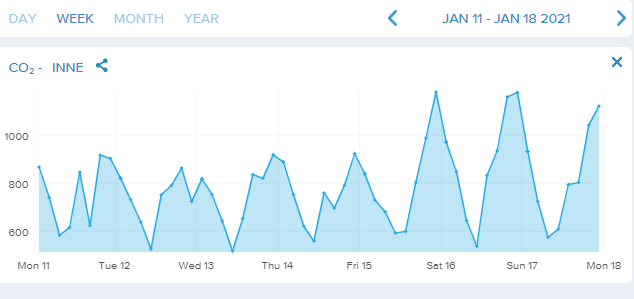
Last weeks CO2 concentrations in our flat. Note its very good weather here with all doors open and much outside air. The red marking show what´s happen if a 6 year old grandson sleep over
My CO2 in the living room during a winter week
Look fot Netatmo Weather stations - their indoor module have a CO2 logger
Sincerely Lasse
pH last week
Last weeks CO2 concentrations in our flat. Note its very good weather here with all doors open and much outside air. The red marking show what´s happen if a 6 year old grandson sleep over
Sincerely Lasse
I am going to see if I can avoid agitation but keep my sine wave working from my wave pumps.
the line is about 10 feet.
kalkwasser is infinitely better and can be controlled.
I am doing all this to prove that CO2 is my problem. But I am still not 100% convinced. Winter without Kalkwasser had similar numbers though. I need to get my indoor/outdoor CO2 meter to see what’s going on.
10 feet doesn't seem bad, mine is much longer and about double the width.
All kalk is doing is binding with the co2 to reduce it (over simplification) and hence less co2 higher pH occurs. Also of course increases your alkalinity and calcium levels so you can't do it forever persay without a method of export. Overdose can also occur where I have never heard of an overdose of gas exchange.
I have never had a problem in winter with the outdoor skimmer line and seen it get down to -5F. Believe I even have a photo in my build thread of it being that cold, I saw no change in tank tempature that the heater was not able to handle, and nothing odd with the skimmer during that cold a period.
I have the same experiencesI have never had a problem in winter with the outdoor skimmer line and seen it get down to -5F. Believe I even have a photo in my build thread of it being that cold, I saw no change in tank tempature that the heater was not able to handle, and nothing odd with the skimmer during that cold a period.
Sincerely Lasse
- Joined
- May 17, 2020
- Messages
- 1,371
- Reaction score
- 2,127
I think there is an added challenge with nanos because the skimmers tend to be underpowered. When I tried a CO2 scrubber there was only minimal if any improvement and it also degraded the effectiveness of my skimmer. Tiny skimmers make a lot of the typical solutions less effective. Right now I'm finding the most effective thing is to open a window and run the bathroom exhaust fan at the same time.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,311
- Reaction score
- 63,658
I am doing all this to prove that CO2 is my problem. But I am still not 100% convinced. Winter without Kalkwasser had similar numbers though. I need to get my indoor/outdoor CO2 meter to see what’s going on.
CO2 is always the problem, if pH is really low and alkalinity is normal or high. There's no other possibility. Knowing any two of pH, alkalinity, and CO2 allows the third one to be calculated mathematically.
The only issue is whether the excess CO2 is coming from elevated CO2 in the room air, or not enough tank aeration to equilibrate the tank water with the room air. In a reef tank low pH is usually caused by the former.
CO2 is always the problem, if pH is really low and alkalinity is normal or high. There's no other possibility. Knowing any two of pH, alkalinity, and CO2 allows the third one to be calculated mathematically.
The only issue is whether the excess CO2 is coming from elevated CO2 in the room air, or not enough tank aeration to equilibrate the tank water with the room air. In a reef tank low pH is usually caused by the former.
It’s just shocking to think that even a small skimmer, with a recirculating CO2 scrubber, with air pulled from outside, doesn’t have the ability to overcome the surface area of a 396 square inches, and at most makes minimum impact.
That’s why I was baffled and thought this is occurring from a biological process of decay. Not dead animals, but bacteria and alike.
Makes me wonder the air volume required to be injected from a skimmer VS the surface area of the tank to make a meaningful impact. I saw an positive impact from the aeration of water test, but I wonder if one could make a formula to guide a sizing recommendation to picking a proper size skimmer.
I do not think that anyone here has stated that it can´t be a biological process of decay that it is - at least in part - responsible for the low pH. The only for sure here is that this statement is true. (my bold)
Do you add organic carbon (vodka or similar Dissolved Organic Carbon)?
Sincerely Lasse
The CO2 that is your problem can be produced of a biological process in your aquarium or could be of external origin (your family breathing in your house and an unicifient ventilation). I think there was some tips how to investigate which source dominate in each aquarium earlier in this thread. CO2 is one of the major waste compounds from bacterial decay and will not affect the alkalinity of your water regardless source.CO2 is always the problem, if pH is really low and alkalinity is normal or high
Do you add organic carbon (vodka or similar Dissolved Organic Carbon)?
Sincerely Lasse
I do not think that anyone here has stated that it can´t be a biological process of decay that it is - at least in part - responsible for the low pH. The only for sure here is that this statement is true. (my bold)
The CO2 that is your problem can be produced of a biological process in your aquarium or could be of external origin (your family breathing in your house and an unicifient ventilation). I think there was some tips how to investigate which source dominate in each aquarium earlier in this thread. CO2 is one of the major waste compounds from bacterial decay and will not affect the alkalinity of your water regardless source.
Do you add organic carbon (vodka or similar Dissolved Organic Carbon)?
Sincerely Lasse
I do on occasion add an acetic acid compound. Though it’s only every few days and .20 mL for this 100 L tank.
I use 20 mL of All For Reef, which is Calcium formate.
build up in the air from family — As much as I’d love to open the doors and windows up, to enjoy fresh air. I live in the Southern US by the cost. So not exactly great weather for that solution.
These pH problems are worth solving. People and the oceans have better success with proper pH. Though I think the easiest solution is to default to Kalkwasser at this point.
These two compounds will speed up the heterotrophic bacteria process, hence create some more internal CO2 (at a given time frame) compared with not use them. If it is a major or minor problem in your case - I have no clue.
Is it possible to cover the DT surface from ambient air for a day or two and let the skimmer manage the whole gas exchanging with outside air? Note what´s happen with pH.
Sincerely Lasse
Is it possible to cover the DT surface from ambient air for a day or two and let the skimmer manage the whole gas exchanging with outside air? Note what´s happen with pH.
Sincerely Lasse
It’s just shocking to think that even a small skimmer, with a recirculating CO2 scrubber, with air pulled from outside, doesn’t have the ability to overcome the surface area of a 396 square inches, and at most makes minimum impact.
I am not aware of any tank that does not have a swing during the day and night even if they are above 8 PH. Skimmers just don't provide perfect gas exchange unfortunately for us. On my tank I still see the swings from 7.9/8 to 8.2 where 7.9/8 is the low before the photo period and 8.2 at the end of the photo period where alage and other things are consuming the tank co2.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,311
- Reaction score
- 63,658
It’s just shocking to think that even a small skimmer, with a recirculating CO2 scrubber, with air pulled from outside, doesn’t have the ability to overcome the surface area of a 396 square inches, and at most makes minimum impact.
That’s why I was baffled and thought this is occurring from a biological process of decay. Not dead animals, but bacteria and alike.
Makes me wonder the air volume required to be injected from a skimmer VS the surface area of the tank to make a meaningful impact. I saw an positive impact from the aeration of water test, but I wonder if one could make a formula to guide a sizing recommendation to picking a proper size skimmer.
Well, the proof is in the pudding, as they say. Volume would be an impossible calculation unless you also knew the completeness of the CO2 exchange as the air passed through the skimmer. CO2 exchange is not instantaneous. Slower than O2.
Ok so I'd like to take my foot out of my mouth on this one. Seeing another question on diy all for reef made me wonder. So turns out their k+ does not stand for potassium stands for cation...weirdo's. A for anion. When you said diy I thought you actually reacted your own chemicals. Its their kit am I right? I can still say it is possible to be out of balance with their part A companies make mistakes sometimes. Its possible that when it is mixed it could be acidic making your tank want to go towards neutral. At the same time the boron in it could be contributing to alkalinity. There is more things than carbonate that can make alkalinity. Borate, hydrogen sulfide via anaerobic decomp, phosphorus...many things can. Can't be certain though they don't list exact ingredient amounts. Check ph of mix with your rinsed in di water probe.
Ok so I'd like to take my foot out of my mouth on this one. Seeing another question on diy all for reef made me wonder. So turns out their k+ does not stand for potassium stands for cation...weirdo's. A for anion. When you said diy I thought you actually reacted your own chemicals. Its their kit am I right? I can still say it is possible to be out of balance with their part A companies make mistakes sometimes. Its possible that when it is mixed it could be acidic making your tank want to go towards neutral. At the same time the boron in it could be contributing to alkalinity. There is more things than carbonate that can make alkalinity. Borate, hydrogen sulfide via anaerobic decomp, phosphorus...many things can. Can't be certain though they don't list exact ingredient amounts. Check ph of mix with your rinsed in di water probe.
Yes the minor and trace elements naming is confusing. I don’t understand the marketing behind the name. So liquid All For Reef can be made via the individual ingredients that Tropic Marin produces. It’s called DIY, but it’s not REALLY diy. It’s still all of the manufacturers components. You are just buying them in bulk and not paying for water. There is a cost savings. As for the performance, I dose 21 mL per 24 hour period in equal parts set by the Apex DOS. The alkalinity is rock solid at 8.6 dKH with no spikes. It takes days to readjust which means corals growing.
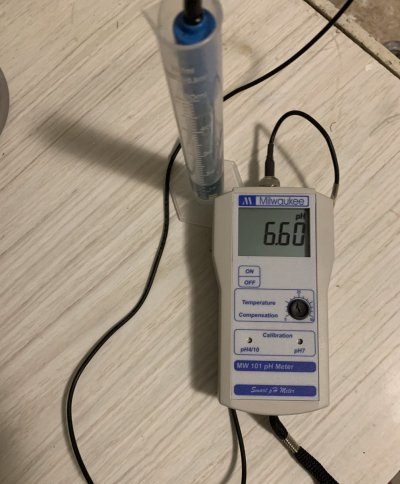
As far as pH. It’s almost neutral. 6.6
If anything it’s not the pH itself, it’s the carbon it attributes to the bacteria that I still think is my main influencer.
Attachments
Similar threads
- Replies
- 2
- Views
- 113
-
- AMS: Article
- Replies
- 61
- Views
- 4,094
-
- AMS: Article
- Replies
- 34
- Views
- 2,546