- Joined
- Mar 20, 2020
- Messages
- 531
- Reaction score
- 439
Did you find instructions on how much AF mineral salt to use per gallon by itself? Everything I read was that mixture in Mag/Calc but I guess I never looked deep enough for it. I am using TMP for the balling but have some af I wouldnt mind using.Many thanks Randy, I will update this thread periodically with my findings. I have set out below what I am going to use, my current aquarium setup, dosage and water parameters so people can follow the progress.
My Aquarium
Please see my aquarium build for information on my Nano (I must update that thread with some newer information!). So people do not have to read the whole thread, I have included the core components here -
Dimensions
10mm glass opti-white (starphire) glass
Width Height Depth Volume External 115cm (45.28") 30cm (11.81") 30cm (11.81") 103.5 litres
(27.3 U.S. gallons)Internal 113cm (44.49") 28cm (11.02") 28cm (11.02") 88.6 litres (23.4 U.S. gallons.)
Equipment
Lighting 2 x TMC Reef-Photon 84w Pumps 2 x Tunze 9040 Pump Battery Backup 2 x Tunze Safety Connecter
2 x Lucas LSLA20-12 12V 20AH Sealed Rechargeable Battery
2 x Maypole 12v 7423A Battery ChargerSkimmer Tunze 9004 DC
(connected to a C02 scrubber)Heater 2 x Aquael Ultra Heater 100w (I think these are branded Cobalt in the US) Heater Battery Backup PowerWalker UPS Doser Coralbox & Apex DOS Computer Controller Neptune Apex 2016
2 x EB6 (UK energy bar)Aqara Hub Water sensor (detect if dosers leaks)
Current Dosing (before switching to Sodium Hydroxide)
Parameter Type Dosage Alkalinity Soda Ash (Randy Holmes-Farley recipe) 11ml over 24 hours Calcium Calcium Chloride (Randy Holmes-Farley recipe) 11ml over 24 hours Trace Aquaforest Reef Mineral Salt 11ml over 24 hours Kalkwasser Calcium Hydroxide 275ml over 12 hours
(when lights are off)
Key Water Parameters
These water parameters were taken before dosing Sodium Hydroxide and will be the key parameters I will closely monitor throughout my journey.
Parameter High Low pH 8.01 - 8.06 7.84 - 7.91 C02 1635 409 Alkalinity 10.02 8.6 Calcium 455 470 Magnesium 1470 1350
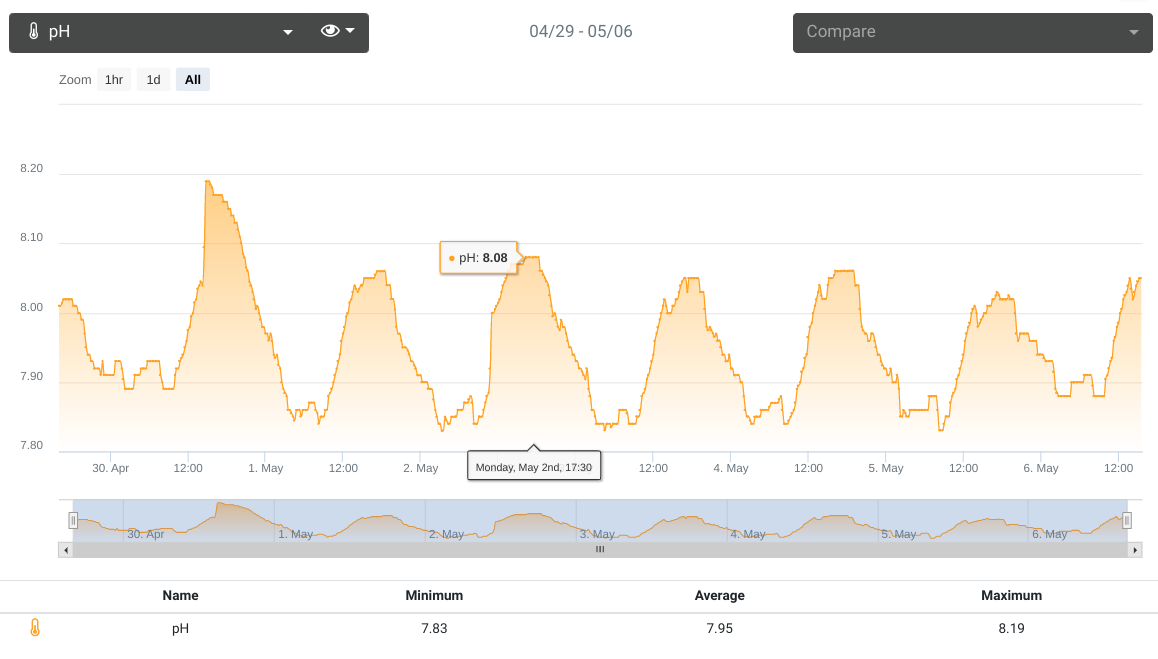
pH
Normally between 7.85 - 8.02. As it has been getting warmer here in the UK, we have had the windows open a little bit more which driving down the C02 in the house and raising the pH in the aquarium. The low reading indicates night time. It is worth noting that I run a C02 scrubber on the Tunze 9004 skimmer inlet. The high of nearly 8.20 is was caused by a large water change.
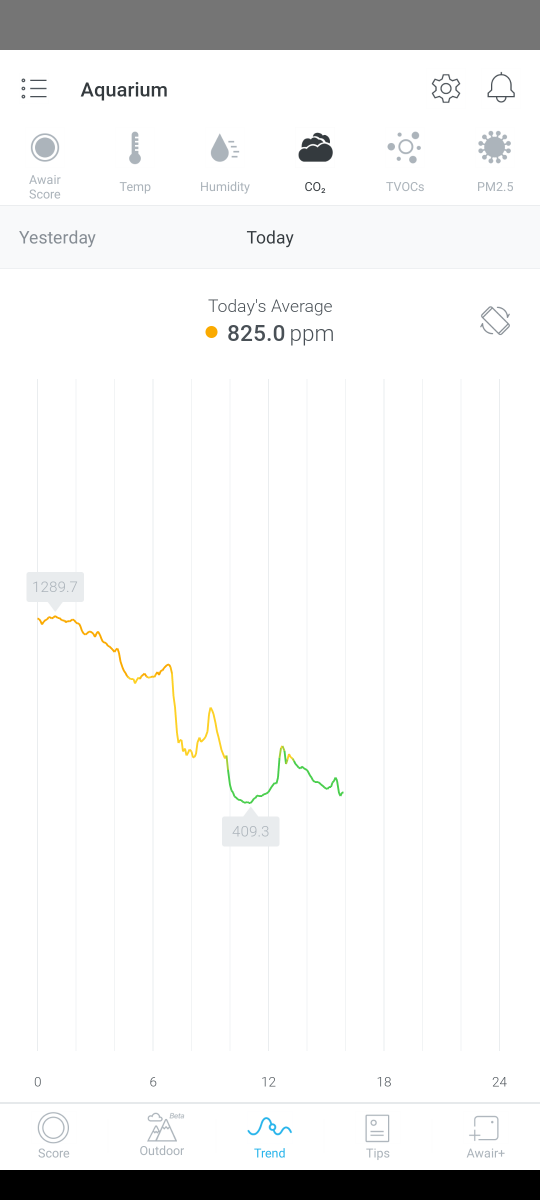
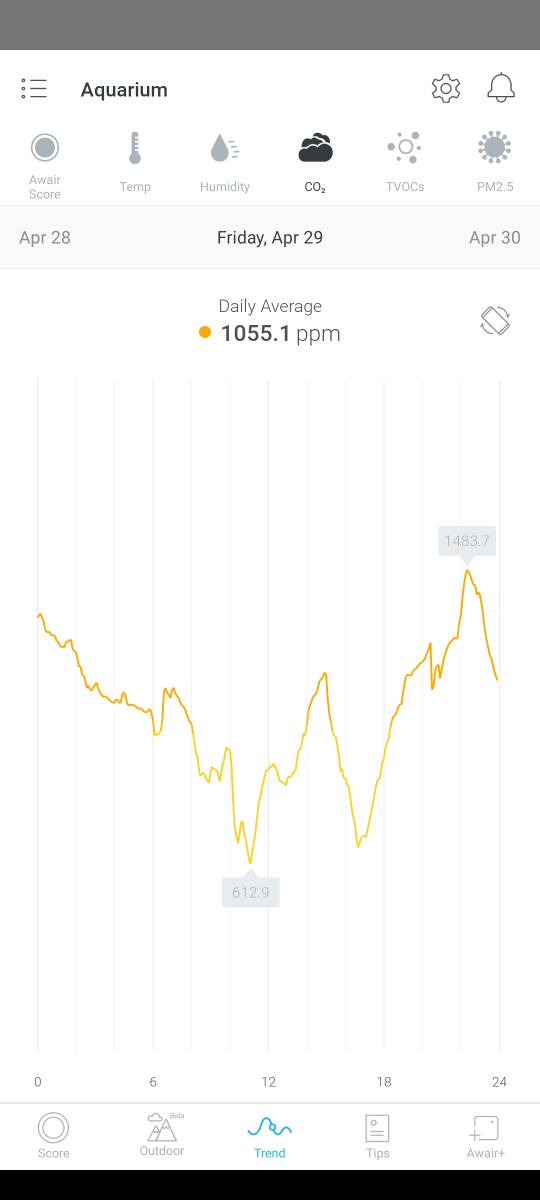
C02
As mentioned above the C02 level in the house has dropped quite a lot during the day with the windows now open but you can see from a couple of examples below that during the evening and night, C02 levels rise which is another contributor to my low pH levels. Even with the Kalkwasser dosing, the pH drops rapidly after lights out.
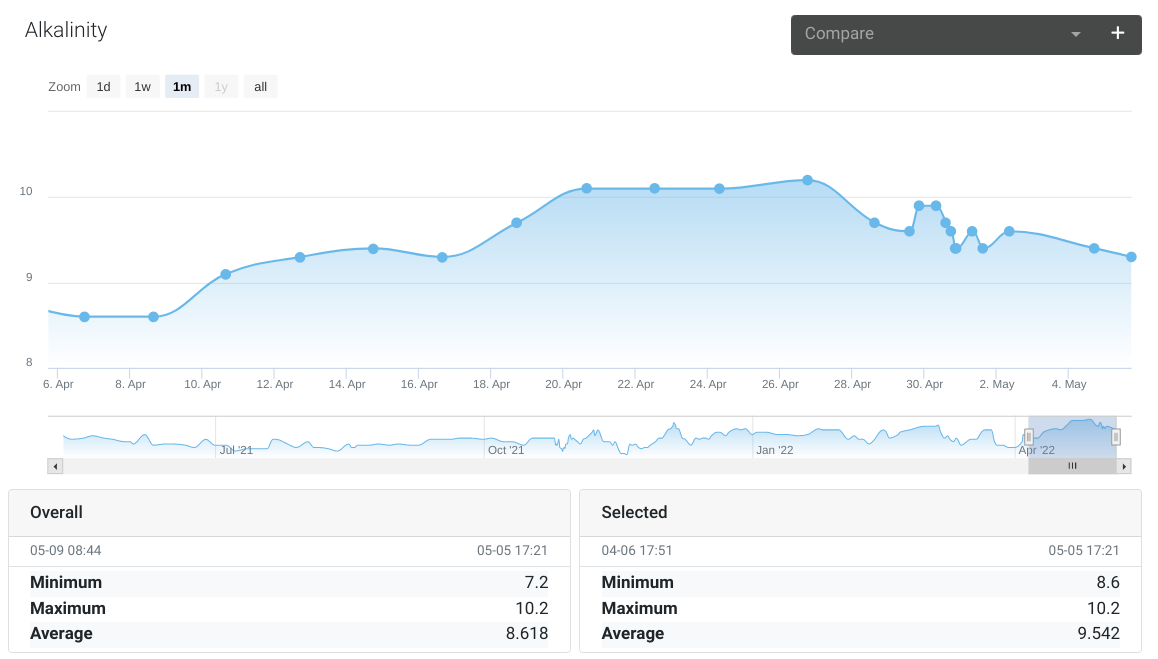
Alkalinity
Normally measures around 9.5 - 10.0 using a Salifert test kit
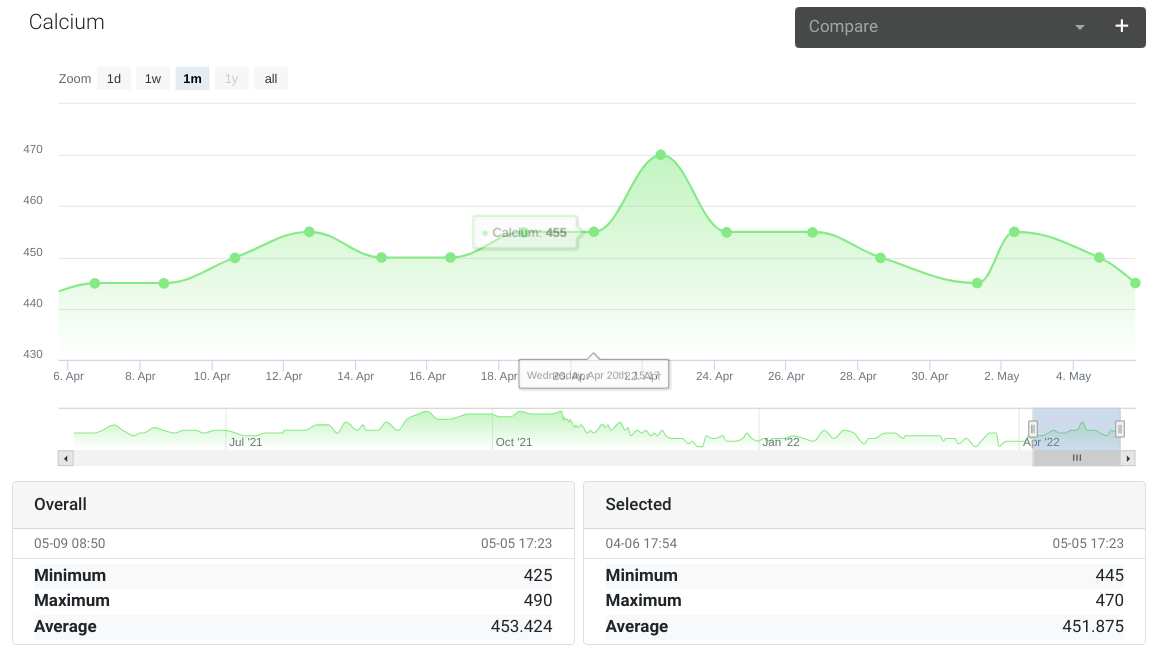
Calcium
Normally measures around 450 using a Salifert test kit
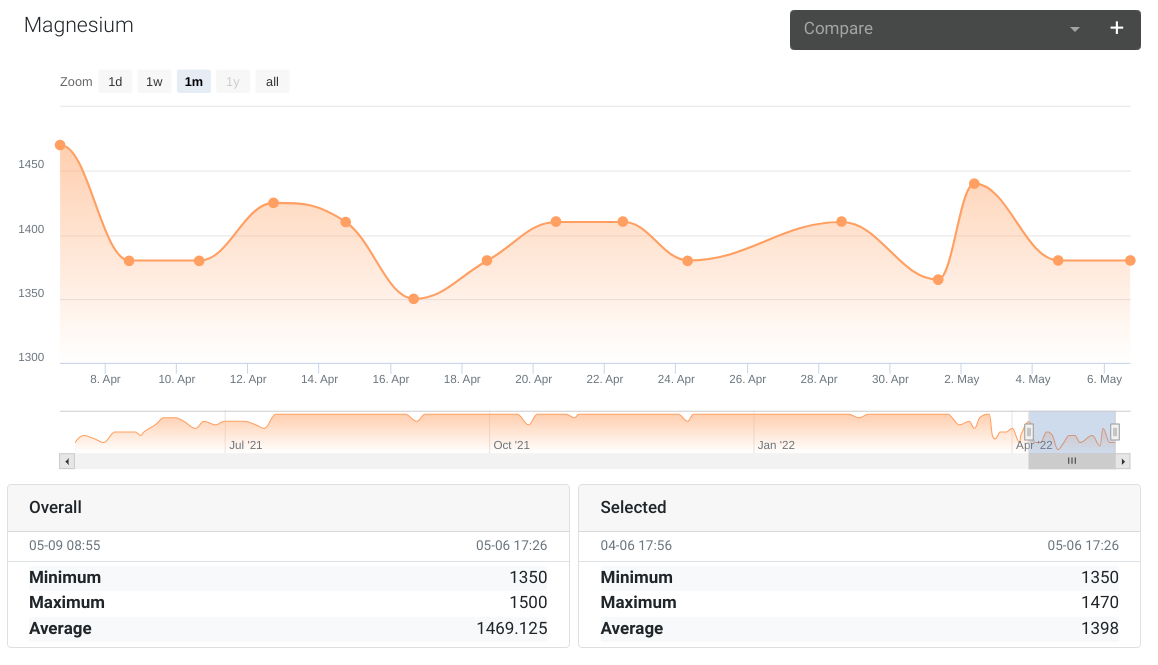
Magnesium
Normally measures around 1400 using a Salifert test kit
New 2 Part
As I dose using a 3 part bailing method, I will be dropping the Magnesium from Part 1 and the Sodium Sulphate from Part 2. I will monitor my Magnesium levels and if I find I need it, I will dose Magnesium to my Part 3 as this is what Aquaforest recommends when using their Mineral Salt (Part 3). Here is what I am about to use -
Part 1 - The Calcium Part (excludes Magnesium)
Dissolve 500 g of calcium chloride dihydrate in enough water to make a total volume of one gallon.
Part 2 - The Alkalinity Part (excludes Sodium Sulfate)
Dissolve 282.8 g of Sodium Hydroxide in enough water to make a total volume of one gallon.
Part 3 - Aquaforest Mineral Salt Part
As per manufacturer's instructions
Therefore, the only change I will be making is swapping out from my current dosing is the Soda Ash with Sodium Hydroxide.
I am expecting the Sodium Hydroxide to arrive tomorrow (Saturday) so I will start posting the results shortly. I will also report back on the following as I have read some people report issues -
- Any issues with containers disintegrating
- Any issues with dosing pipes discoloration or disintegrating
- Sediment on the glass bottom
Hope people find this interesting and a BIG thank you to Randy for all the advice and solutions he offers.



















