- Joined
- Jan 22, 2020
- Messages
- 304
- Reaction score
- 238
I started a new tank recently and waited 8 months to hook up my trident because I didn’t see a reason to waste reagent without much in the tank. I use HW-Marinemix Reefer which according to BRS tested at 9 dKH, 450 Calcium, 1380 Magnesium. I used the reef calculator to find out how much Sodium Carbonate to bring Alk up to 7 Then kept adding to bring it up to 7.5. Over the course of 5 weeks I’ve added 25 grams of Sodium Carbonate and the Ca and Mag have slowly come down. Twice in the last few weeks I had added 2 grams Calcium Chloride to add some Chloride and read from Randy that it shouldn’t cause Calcium to rise much somewhere. Today the Mag and Ca match what the salt is supposed to mix up at, but I am confused why I have had to add so much Sodium Carbonate to raise the Alk.
I have my tank in my basement tank room and the PH had been running around 7.9, but started dropping even more 7.4 when we were home during the day during the shutdown. I ran 1 inch from outside to feed my skimmer and this corrected the PH issue and now it rides between 8.1- 8.2. Could the high CO2 that drove down the PH for those months somehow have created the lower alkalinity I’ve been dealing with?
I am concerned that I am creating an imbalance of Sodium ion to Chloride ion. I did not want to dose Calcium Chloride “2 part” when what I was trying to do was raise the Alk, not the Calcium or Magnesium with their Chloride salts. All of the LFS that I buy from run their Alk between 8-9 which is one of the reasons I would like to get my Alk higher and so that when I do water changes my parameters match and I’m not creating Alk swings. What advice does anyone have? Am I imagining a problem with imbalanced ions? Is there a way to add Chloride without adding Calcium or Magnesium? @Randy Holmes-Farley @ReefSquad
I would really like to make the move to being able to grow some SPS as I have not had any success with them which is why the need to understand more about my water chemistry and controlling etc. Thanks for any advice in advance.
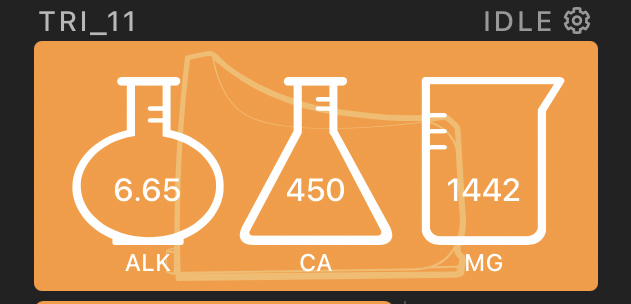
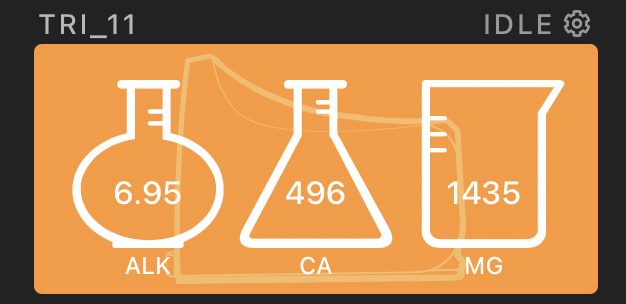
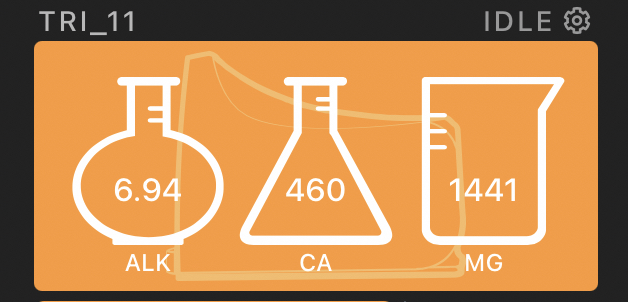
I calibrated the Trident after letting it run for about 11 days and it matched the calibration fluid perfectly.
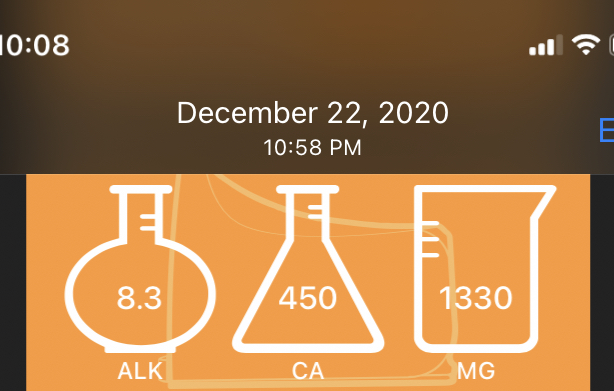
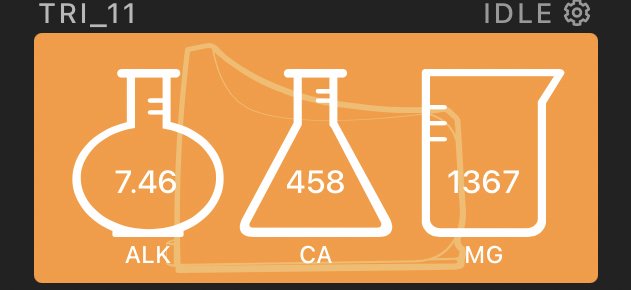
Today it tested around 7.5, 450, 1370.
I have my tank in my basement tank room and the PH had been running around 7.9, but started dropping even more 7.4 when we were home during the day during the shutdown. I ran 1 inch from outside to feed my skimmer and this corrected the PH issue and now it rides between 8.1- 8.2. Could the high CO2 that drove down the PH for those months somehow have created the lower alkalinity I’ve been dealing with?
I am concerned that I am creating an imbalance of Sodium ion to Chloride ion. I did not want to dose Calcium Chloride “2 part” when what I was trying to do was raise the Alk, not the Calcium or Magnesium with their Chloride salts. All of the LFS that I buy from run their Alk between 8-9 which is one of the reasons I would like to get my Alk higher and so that when I do water changes my parameters match and I’m not creating Alk swings. What advice does anyone have? Am I imagining a problem with imbalanced ions? Is there a way to add Chloride without adding Calcium or Magnesium? @Randy Holmes-Farley @ReefSquad
I would really like to make the move to being able to grow some SPS as I have not had any success with them which is why the need to understand more about my water chemistry and controlling etc. Thanks for any advice in advance.
I calibrated the Trident after letting it run for about 11 days and it matched the calibration fluid perfectly.
Today it tested around 7.5, 450, 1370.















