- Joined
- Jun 5, 2019
- Messages
- 446
- Reaction score
- 324
Here it is!
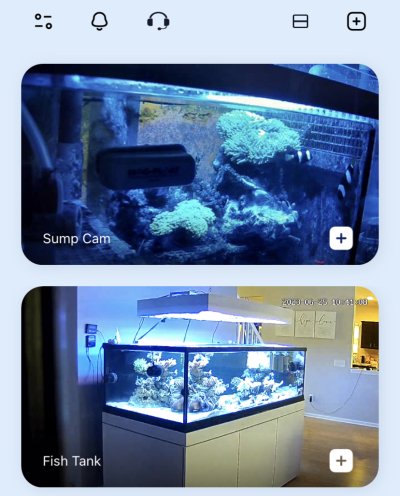
Under daylight from the top:

Through the glass under blues:

And as you can see the idea is to try and get the milii to host in a new anemone. The had readily hosted in the mertensii that killed everything at the start of this thread. On the principle alone I had to get rid of that anemone so I did. It was always a bit of a malcontent, would be perfectly fine for weeks and then reposition itself. I have no idea why this was - couldn’t be light as there was a wall hammer and maxima literally as happy as a clam
 right beside it. My guess is flow but not for certain. Either way I am happy to be rid of it.
right beside it. My guess is flow but not for certain. Either way I am happy to be rid of it.
As far as hosting, the milii are a bit of an enigma. There’s not a lot of research out there on them, and what is out there is somewhat of guesswork. Some wild photos suggest the ubiquitous BTA which for a variety of reasons I’d rather not keep. Some other postings I found on the few wild photos were guessing other Heteractis species like malu or crispa which I believe are both sand dwelling. I didn’t see any mention of supposed hosting of H. aurora and am not even sure where to source one if they did. I have never kept any of these before and would rather not do a container of sand in the sump that would be needed for most/all of them.
I tried putting my gigantea in the sump with them for a couple days and they had zero interest. Like other clarkii family clowns, I am guessing they would also host a haddoni, but again with the sand issue. So that leads me back to the only other Heteractis anemone on the list - the mag. So far they haven’t dropped straight into it but they aren’t actively avoiding it like they did with the gig. You’ll have to ignore the cloudy water - had just done some maintenance in the sump.
So not hosting in it so far, but fingers crossed they will come along.

Under daylight from the top:

Through the glass under blues:

And as you can see the idea is to try and get the milii to host in a new anemone. The had readily hosted in the mertensii that killed everything at the start of this thread. On the principle alone I had to get rid of that anemone so I did. It was always a bit of a malcontent, would be perfectly fine for weeks and then reposition itself. I have no idea why this was - couldn’t be light as there was a wall hammer and maxima literally as happy as a clam
As far as hosting, the milii are a bit of an enigma. There’s not a lot of research out there on them, and what is out there is somewhat of guesswork. Some wild photos suggest the ubiquitous BTA which for a variety of reasons I’d rather not keep. Some other postings I found on the few wild photos were guessing other Heteractis species like malu or crispa which I believe are both sand dwelling. I didn’t see any mention of supposed hosting of H. aurora and am not even sure where to source one if they did. I have never kept any of these before and would rather not do a container of sand in the sump that would be needed for most/all of them.
I tried putting my gigantea in the sump with them for a couple days and they had zero interest. Like other clarkii family clowns, I am guessing they would also host a haddoni, but again with the sand issue. So that leads me back to the only other Heteractis anemone on the list - the mag. So far they haven’t dropped straight into it but they aren’t actively avoiding it like they did with the gig. You’ll have to ignore the cloudy water - had just done some maintenance in the sump.
So not hosting in it so far, but fingers crossed they will come along.