hello, does anyone know the mg/l concentration of the triton reagents?
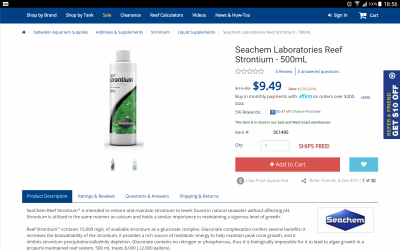
take for example triton strontium. seachem strontium states 10,000 mg/l, on their bottle. what's the triton value?
does anyone have a complete (or partial) list for the triton reagents/additives? i can't find anything on triton's website.
***********
take for example triton strontium. seachem strontium states 10,000 mg/l, on their bottle. what's the triton value?
does anyone have a complete (or partial) list for the triton reagents/additives? i can't find anything on triton's website.
***********