I can agree to that testing and too much playing around to fix something could be bad. Looks can be deceiving though. I guess it would depend on what is going on. I would try to fix it if I could, or at least test again in 4 - 8 hours to see if its resolving itself. It would get me to start looking for a problem like you said. You see example I gave in another thread 82 snails dead. That was at .5mg levels and less. Well long story short nitrite was the problem, fixed it, and every thing is ok now. I see similar instances on here where urchins slow, snails dying. I suspect nitrite problem. I suppose for some it maybe hard to id a problem. The best thing I can suggest would be for people to educate themselves on things that could be a problem, and yes fix if possible.Would you do something different - if everything in the tank looks good?
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
My thoughts on nitrite.
- Thread starter DrZoidburg
- Start date
- Tagged users None
You got a link to the dead snails thread, please. Not actually seen any evidence before. CheersI can agree to that testing and too much playing around to fix something could be bad. Looks can be deceiving though. I guess it would depend on what is going on. I would try to fix it if I could, or at least test again in 4 - 8 hours to see if its resolving itself. It would get me to start looking for a problem like you said. You see example I gave in another thread 82 snails dead. That was at .5mg levels and less. Well long story short nitrite was the problem, fixed it, and every thing is ok now. I see similar instances on here where urchins slow, snails dying. I suspect nitrite problem. I suppose for some it maybe hard to id a problem. The best thing I can suggest would be for people to educate themselves on things that could be a problem, and yes fix if possible.
@Garf I was referring to comment I made in Lasse's thread. You both commented in there page 5. It wasn't about exactly what was done or how to fix it. It did happen though. To fix the issue it was a simple tweak of a needle valve to slow the flow down, and wait some time before adding new livestock.

 www.reef2reef.com
www.reef2reef.com

The importance of nitrite measurements in a reef aquarium
@Lasse - do you think one of the reasons you disagree is that you have the ability to measure these things in real-time? I mean frankly - I think your nitrite argument makes complete sense. Ammonia I think is a secondary issue. BUT - I do not want to do nitrite tests every day or week or month.
 www.reef2reef.com
www.reef2reef.com
Or - could nitrite be very similar to carbon dioxide in humans? No toxicity is seen at amounts that can be found in natural environments. Only when spiking CO2 to wholly unnatural amounts can you see any issues.Sort of another thing I'm batting at. How do we know that a life time of even intermittent exposures does not cause harm long term, shorter life, disease outbreaks etc.. Only stating it should be tested and adjusted/kept in check respectfully. The nitrite is "non-toxic" community saying based on a limited list of food use fisheries species is not us reef keepers as a whole. Where a few of things we do keep even though studies are limited, can be shown to be effected. What about a comprehensive list of fish, corals, inverts, sponges, or anything so many people keep. No such list exists. People say they care about their pets but ignore possibility, and in some cases near certainty. Why not test occasionally. It's so easy and takes no time. To your last question I believe my answer would be yes it helps. Some reasons we may know why, and some not.
The issue is, as have been explained, you really cannot get more than perhaps 2 ppm nitrite ion in water unless the ammonia level that preceded it was like at 10 ppm, which would have been acutely lethal to the fish and they never would have lived to see the nitrite.
All of the marine nitrite toxicity studies listed in Noga were of spiked samples.
A much more important thing to look for would be chronic toxicity in nitrate-nitrogen.....
Jay
Still disagreeing with this in whole except possible nitrate toxicity. Nitrite I guess would have similar effects to CO2 but I don't think the two are comparable. As well as other effects aside from asphyxiation it has. If something damages nitrate bacteria, it can get more than 2ppm, and other reasons. We have seen some examples on here go as high as 10ppm NO2 and higher. One I provide to you at .5mg and less in the case of particular snails. I have not personally crunched the numbers but if this is trueOr - could nitrite be very similar to carbon dioxide in humans? No toxicity is seen at amounts that can be found in natural environments. Only when spiking CO2 to wholly unnatural amounts can you see any issues.
The issue is, as have been explained, you really cannot get more than perhaps 2 ppm nitrite ion in water unless the ammonia level that preceded it was like at 10 ppm, which would have been acutely lethal to the fish and they never would have lived to see the nitrite.
All of the marine nitrite toxicity studies listed in Noga were of spiked samples.
A much more important thing to look for would be chronic toxicity in nitrate-nitrogen.....
Jay
@Jay Hemdal Then starting at 10ppm ammonia and getting 2 ppm +/- nitrite is not true. Spiked or natural is irrelevant. It would not get lethal to some things. Others it could, and it also could cause irreversible effects.For every ppm addition of NH3/NH4 around 2.6 ppm nitrite will be formed (1 ppm NH4 = 14/18 (0.78) ppm NH4-N -> 0.78 ppm NO2-N = 0.78 x 46/14 (3.29) NO2 = 2,6 ppm NO2)
How in the whole world is the LC50 test done? I have seen upbuild NO2 over 10 in waters with low measurable ammonia after a bad working biofilter in fish farms. it is depending on the loadyou really cannot get more than perhaps 2 ppm nitrite ion in water unless the ammonia level that preceded it was like at 10 ppm,
If 10 ppm NH4 with the molecule weight of 18 (the N part 14) it correspond to 10*14/18 ppm NH4-N
= 7,77 ppm (NH4-N) - if this (as it can in a new aquarium) be total converted to NO2-N its equal to 7.77 NO2-N. 7.77 NO2-N = 7.77*(14+16*2)/14 = 7.77*3,29 = 25.53 ppm NO2
Sincerely Lasse
Granted naturally the animals get acclimated to these levels slower than spiked. However if high enough could cause problems imo.
I thought the nitrogen atom was one to one? The equation I’ve used is: NH4+OH- + 1.5 O2 = H+ NO2- + 2H2O Then because the NO2 is turned to NO3, it never gets as high as the original ammonia level has been. Typically, if I see 0.50 ammonia, the corresponding nitrite level peaks at around 2How in the whole world is the LC50 test done? I have seen upbuild NO2 over 10 in waters with low measurable ammonia after a bad working biofilter in fish farms. it is depending on the load
If 10 ppm NH4 with the molecule weight of 18 (the N part 14) it correspond to 10*14/18 ppm NH4-N
= 7,77 ppm (NH4-N) - if this (as it can in a new aquarium) be total converted to NO2-N its equal to 7.77 NO2-N. 7.77 NO2-N = 7.77*(14+16*2)/14 = 7.77*3,29 = 25.53 ppm NO2
Sincerely Lasse
Jay
@Jay Hemdal Ammonia and the others though is a gas in water I do believe. It would be better to exclude water from weight equation. Would make sense nitrite/nitrate would be higher levels on test kit hydrogen weighs 1, oxygen about 16. Though the nitrogen maybe one to one the actual amount of compound would be different. I think he means 7.77mg is the Nitrogen of 10 ppm ammonium. I get about 25 mg/L Nitrite also (rough calculation)
What??????I thought the nitrogen atom was one to one? The equation I’ve used is: NH4+OH- + 1.5 O2 = H+ NO2- + 2H2O Then because the NO2 is turned to NO3, it never gets as high as the original ammonia level has been. Typically, if I see 0.50 ammonia, the corresponding nitrite level peaks at around 2
Jay
N have the atomic weight of 14. Hydrogen have the atomic weight of 1 Oxygen the weight of 16. The weight of the NH4 molecule is therefore 18 (14+4) and the nitrite molecule will have an atomic weight of 46 It means that the nitrite molecule is around 2.5 times heavier.
If you start with 0.5 mg/l (0.05 g NH4 to 100 L) ammonium and all turn to nitrite - the N content is 14/18 * 0.5 = 0.39 mg/L It normally is expressed as NH4-N. And in this your right the NH4-N weight is the same as the NO2-N weight. The contribution of N to the weight in the whole molecule is the same in both NH4 and NO2. With other words 0.39 mg/L of NH4-N will be 0.39 mg/L NO2-N. But we are talking of the weight of the total NO2 molecule and therefore we need to add the weight of the oxygen to the calculation, hence 0.39 mg/L NO2-N is 0.39*46/14 = 0,39*3.29 = 1.28 expressed as NO2. In most - especially older toxicological literature - and in most of our hobby tests - the concentration is expressed as the whole molecule - in this case NO2. In environmental and when a mass balance is important - it is normal to express the concentration as nitrogen nitrite (NO2-N) In our case - toxicological considerations - we are interested of the whole molecule (it is that which is the important and toxic - not the N or O - just the combination. Compare NH4 and NH3 there is should in a toxicological point of view be total meaningless to use NH3-N as the measurement. Most litterature and testset use NO2 as nomenclature. There is some that use NO2-N and it create a lot of misunderstandings.
I will take an example. We start with a 100 L aquarium (if you want to understand the basics of this - you need to use the metric system) 100 L is around 26.42 gallon. The instruction says that we should start with 1 mg/L (nearly the same as ppm) ammonia (I use NH4 as example here) This means that I need to add 0.1 g of NH4 into the 100 L. Thats means when ammonia reads 0 - around 0.1*14/18 = 0.77 N has be transferred to nitrite - the NO2- N is 0.77 But the NO2 is 3.29*0.77 = 2.5 mg/L NO2 (ppm NO2). Now - the process stall - as it could and the aquarist that follow instructions add another 1 ppm ammonia on day 3 - because the second step is stalled 2.5 mg/L of NO2 will add to the other 2.5 and you are up in 5 mg/L of NO2.
I have seen advises here to start with 4 ppm ammonia, following up with more and confirm the first step with more addition of ammonia. All of this without measuring NO2. In worst case if you to a 100 l aquarium have started with 4 ppm ammonia (0.4 g of ammonia to 100 L). adding another 0.4 g at day three and control the first step with another
0.4 g - the total amount will be 1.2 g ammonia or 1.2*14/18 = 0.93 g NH4-N to the aquarium an in worst case it will be 0.93 g of NO2-N in 100 L -> 9,3 mg/L NO2-N. 9.3 mg/L NO2-N is 3,29*9,3 = 30.6 mg/L (ppm) NO2. This is in worst case with a stalled second step - which means no or very small amount of NO2 converted to NO3. and this is a very common scenario when you start a nitrification cycle with high addition of ammonia - IMO
To this I can add that we are in a pH around 8 - 8.5 and some free ammonia will be formed (NH3) Free ammonia itself have been shown to suppress the second step at least in freshwater but in higher concentrations that we normal have. However - with the methods to add ammonia in saltwater aquariums start up - I have seen tons of reports that indicate stalled nitrification cycling process, And with stalled I mean that the second step NO2-NO3 not working properly.
I hope this will clarify why I have the standpoint I have. Nitrite measurements is the key factor in order to know if the whole nitrification cycle is working in a proper way or not. IMO if possible - no living creatures should be added before it is completed and seamless and 0.1 ppm NO2 is a good threshold. However there is methods to start with low amounts of daily contribution of ammonia - like my 15 steps - (an slow rising of the amount) and/or adding biofilm or/and photosynthetic organisms early in the start up that hinder the stall of the cycle and that you never will see a nitrite peak,
Sincerely Lasse
4 word tree octopus and flat earthAnecdotes are not totally useless. I knew from experience that carbon causes HLLE. I determined this as far back as 1998 through careful observation. I'd get in arguments with people all the time over it. Finally, out of frustration, a decade later, I ran my study which showed the trenchant effect of carbon on surgeonfish.
The converse is also dangerous - overextrapolation is rampant. People read a peer-reviewed paper and apply it to slightly different circumstances and it doesn't hold up. The best case of that is the "76 day survivorship of Cryptocaryon tomonts". Published by Colorni and Burgess, it was picked up by a person and widely disseminated as gospel. Trouble is, this study used survivorship in a xeric culture (no bacteria) under low temperatures. The author himself said this won't apply to actual aquariums - yet here we are, battling that misinformation.
Jay
I see, but we never see nitrite levels anywhere near that. I think I may have an answer to my confusion: For 30 years, I've made my measurements with a spectrophotometer it measure ammonia as NH4, but nitrite is reported at Nitrite-N, as is Nitrate. I knew that the conversion factor for Nitrate-N is 4.4x, but I never bothered with converting Nitrite-N since it is so transient, and only seen during a portion of the tank run in.@Jay Hemdal Ammonia and the others though is a gas in water I do believe. It would be better to exclude water from weight equation. Would make sense nitrite/nitrate would be higher levels on test kit hydrogen weighs 1, oxygen about 16. Though the nitrogen maybe one to one the actual amount of compound would be different. I think he means 7.77mg is the Nitrogen of 10 ppm ammonium. I get about 25 mg/L Nitrite also (rough calculation)
Jay
Lasse,What??????
N have the atomic weight of 14. Hydrogen have the atomic weight of 1 Oxygen the weight of 16. The weight of the NH4 molecule is therefore 18 (14+4) and the nitrite molecule will have an atomic weight of 46 It means that the nitrite molecule is around 2.5 times heavier.
If you start with 0.5 mg/l (0.05 g NH4 to 100 L) ammonium and all turn to nitrite - the N content is 14/18 * 0.5 = 0.39 mg/L It normally is expressed as NH4-N. And in this your right the NH4-N weight is the same as the NO2-N weight. The contribution of N to the weight in the whole molecule is the same in both NH4 and NO2. With other words 0.39 mg/L of NH4-N will be 0.39 mg/L NO2-N. But we are talking of the weight of the total NO2 molecule and therefore we need to add the weight of the oxygen to the calculation, hence 0.39 mg/L NO2-N is 0.39*46/14 = 0,39*3.29 = 1.28 expressed as NO2. In most - especially older toxicological literature - and in most of our hobby tests - the concentration is expressed as the whole molecule - in this case NO2. In environmental and when a mass balance is important - it is normal to express the concentration as nitrogen nitrite (NO2-N) In our case - toxicological considerations - we are interested of the whole molecule (it is that which is the important and toxic - not the N or O - just the combination. Compare NH4 and NH3 there is should in a toxicological point of view be total meaningless to use NH3-N as the measurement. Most litterature and testset use NO2 as nomenclature. There is some that use NO2-N and it create a lot of misunderstandings.
I will take an example. We start with a 100 L aquarium (if you want to understand the basics of this - you need to use the metric system) 100 L is around 26.42 gallon. The instruction says that we should start with 1 mg/L (nearly the same as ppm) ammonia (I use NH4 as example here) This means that I need to add 0.1 g of NH4 into the 100 L. Thats means when ammonia reads 0 - around 0.1*14/18 = 0.77 N has be transferred to nitrite - the NO2- N is 0.77 But the NO2 is 3.29*0.77 = 2.5 mg/L NO2 (ppm NO2). Now - the process stall - as it could and the aquarist that follow instructions add another 1 ppm ammonia on day 3 - because the second step is stalled 2.5 mg/L of NO2 will add to the other 2.5 and you are up in 5 mg/L of NO2.
I have seen advises here to start with 4 ppm ammonia, following up with more and confirm the first step with more addition of ammonia. All of this without measuring NO2. In worst case if you to a 100 l aquarium have started with 4 ppm ammonia (0.4 g of ammonia to 100 L). adding another 0.4 g at day three and control the first step with another
0.4 g - the total amount will be 1.2 g ammonia or 1.2*14/18 = 0.93 g NH4-N to the aquarium an in worst case it will be 0.93 g of NO2-N in 100 L -> 9,3 mg/L NO2-N. 9.3 mg/L NO2-N is 3,29*9,3 = 30.6 mg/L (ppm) NO2. This is in worst case with a stalled second step - which means no or very small amount of NO2 converted to NO3. and this is a very common scenario when you start a nitrification cycle with high addition of ammonia - IMO
To this I can add that we are in a pH around 8 - 8.5 and some free ammonia will be formed (NH3) Free ammonia itself have been shown to suppress the second step at least in freshwater but in higher concentrations that we normal have. However - with the methods to add ammonia in saltwater aquariums start up - I have seen tons of reports that indicate stalled nitrification cycling process, And with stalled I mean that the second step NO2-NO3 not working properly.
I hope this will clarify why I have the standpoint I have. Nitrite measurements is the key factor in order to know if the whole nitrification cycle is working in a proper way or not. IMO if possible - no living creatures should be added before it is completed and seamless and 0.1 ppm NO2 is a good threshold. However there is methods to start with low amounts of daily contribution of ammonia - like my 15 steps - (an slow rising of the amount) and/or adding biofilm or/and photosynthetic organisms early in the start up that hinder the stall of the cycle and that you never will see a nitrite peak,
Sincerely Lasse
Sorry - I see my confusion now is that I measure Nitrite as Nitrite-N and ammonia as NH4.
Jay
- Joined
- Sep 20, 2018
- Messages
- 1,117
- Reaction score
- 1,090
Why doesn't everyone have a spreadsheet that does the conversions?
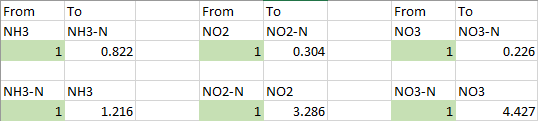
Times was different many years ago - we never started an aquarium with adding tons of chemical ammonia. It still not common in Europe either. I would never put any fish into a tank that started that way (with tons of ammonia) before nitrite show a peak and a gone down below 0.1 ppm NO3.Lasse,
Sorry - I see my confusion now is that I measure Nitrite as Nitrite-N and ammonia as NH4.
Jay
In freshwater the nitrite goes into the body through the gills (chloride cells) and that it can create damage on the cells..
Everything I have read about the way nitrite enter the body in saltwater fish indicate that it enter the fish trough uptake in the digestive tract and leave the body through the same chloride cells and that it also create damage on the chloride cells. At least in a start up - you can´t exclude sublethal damage in these cases IMO.
Sincerely Lasse
- Joined
- Aug 24, 2016
- Messages
- 1,499
- Reaction score
- 2,293
I want to add the following scientific findings and own hyptheses to the discussion:
- In freshwater fish nitrite is taken up through the chloride cells in the gills in competition with chloride ion
- Nitrate is highly enriched in blood plasma of fish and oxidizes hemoglobin to methemoglobin, the most important damage induced by nitrite
- The chloride ion concentration in saltwater is 0,546 M or more than 19 g/L (19380 mg/L), for comparison with Gutierrez et al. who used 200 mg/L
- Nitrite, like nitrate, is a strong oxidant and may oxidize further important stuctures like fatty acids and alter cell membranes in this way
- In marine fish nitrite is not enriched through the chloride cells in gills and has a much less detrimental effect
- Effects of nitrite is well known from freshwater fish, crustaceans and other invertebrates. My hypothesis is that there are no other effects in marine fish and invertebrates and the effect is much reduced due to much lower concentrations in the blood and tissues of marine fish and invertebrates
- In freshwater fish nitrite is taken up through the chloride cells in the gills in competition with chloride ion
- Nitrate is highly enriched in blood plasma of fish and oxidizes hemoglobin to methemoglobin, the most important damage induced by nitrite
- The chloride ion concentration in saltwater is 0,546 M or more than 19 g/L (19380 mg/L), for comparison with Gutierrez et al. who used 200 mg/L
- Nitrite, like nitrate, is a strong oxidant and may oxidize further important stuctures like fatty acids and alter cell membranes in this way
- In marine fish nitrite is not enriched through the chloride cells in gills and has a much less detrimental effect
- Effects of nitrite is well known from freshwater fish, crustaceans and other invertebrates. My hypothesis is that there are no other effects in marine fish and invertebrates and the effect is much reduced due to much lower concentrations in the blood and tissues of marine fish and invertebrates
There could be a different pathway for sublethal (and probably even lethal) damage of nitrite in saltwater compared with freshwater. In saltwater - nitrite is not passing into the body through the chloride cells - it can come into the bloodstream through the digestive tract and is excreted by the chloride cells out from the body - but in both cases nitrite has going through the chloride cells. In fresh water fish (that's not drink) it going in and rise the NO2 concentrations in the blood - in saltwater fish (huge drinkers) - nitrite enter the bloodstream through the digestive tract and actively transported out (and decrease the concentrations in the bloodstream) through the chloride cells. In this they will interact with the osmos balance and contribute to both an energy and osmotic stress. The quote below is from hereI want to add the following scientific findings and own hyptheses to the discussion:
- In freshwater fish nitrite is taken up through the chloride cells in the gills in competition with chloride ion
- Nitrate is highly enriched in blood plasma of fish and oxidizes hemoglobin to methemoglobin, the most important damage induced by nitrite
- The chloride ion concentration in saltwater is 0,546 M or more than 19 g/L (19380 mg/L), for comparison with Gutierrez et al. who used 200 mg/L
- Nitrite, like nitrate, is a strong oxidant and may oxidize further important stuctures like fatty acids and alter cell membranes in this way
- In marine fish nitrite is not enriched through the chloride cells in gills and has a much less detrimental effect
- Effects of nitrite is well known from freshwater fish, crustaceans and other invertebrates. My hypothesis is that there are no other effects in marine fish and invertebrates and the effect is much reduced due to much lower concentrations in the blood and tissues of marine fish and invertebrates
Due to drinking of sea water, in marine fish the main nitrite uptake to blood occurs in the intestine, competing with Clin the Na?/K?/2Cl- and Na?/Cl- cotransporters (Grossel and Jensen 1999). Also, nitrite can reach fish blood by diffusion through the gill epithelium. However, due to high Clconcentration, the active transport of ions in the gill excretes Cl- and Na? and in addition actively excretes the NO2 - instead the Cl- (Jensen 2003).
Further on - In this publication the authors found this - my bold. I can only link to pay wall here but it is possible to find the whole text on internet
4.3. Naþ/KþeATPase In most euryhaline teleosts, NKA (an ATPase that provides energy for ion transportation across membranes and maintains ion cytoplasmic homeostasis) activity exhibits a significant increase with the elevated salinity (Saoud et al., 2007; Jeong et al., 2014), as observed in this study. However, when juveniles were exposed to nitrite, NKA and mRNA levels in most tissues were significantly inhibited at higher salinity. Specifically, there was significant interaction between salinity and nitrite on the response of NKA in the gills, kidney, and intestine. These findings suggested that most energy was used to resist free radicals caused by nitrite, and the osmotic regulation ability was depressed. Previous studies support the argument that NKA is negatively correlated with oxidative stress (Chauhan et al., 2002; Maurya and Prakash, 2013). Therefore, we cannot ignore the role of the ATPase NKA in the mitigating effect of salinity on nitrite toxicity; this ATPase may contribute in the reduction of oxidative stress damage caused by nitrite exposure. Given our results, it may now be appropriate to evaluate whether the increased production of ATP can alleviate damage caused by oxidative stress.
My standpoint is that there is so many questions marks according sublethal effect even in saltwater that we should use the precautionary principle and advise people not to add fish when the whole nitrification cycle is completed. It is good husbandry.
According pathways for uptake of NO2 in marine teleost - here
Sincerely Lasse
Last edited:
- Joined
- Aug 24, 2016
- Messages
- 1,499
- Reaction score
- 2,293
Hi Lasse,
may I quote your link (thank you very much for it!)?There could be a different pathway for sublethal (and probably even lethal) damage of nitrite in saltwater compared with freshwater.
25 mg/L NO2-N is 82 mg/L NO2. I think this concentration is beyond good and evil for reef aquarists.Medeiros et al.:
Based on our results, we recommend to avoid concentrations higher than 0.57 mg/L of NH3-N and 25 mg/L of NO2-N in water.
Hi Lasse,
may I quote your link (thank you very much for it!)?
Yes
They did not gel lower but it was a higher rate of gill damage already there25 mg/L NO2-N is 82 mg/L NO2. I think this concentration is beyond good and evil for reef aquarists.
Further on - interesting is the pathways that is described and the findings about the energy demands in the second article and depression of osmosis regulation.
It seems like the most popular start method in the US is to add huge amount of ammonia, bacteria in a bottle and put in fish after the NH3 is low - not bother for the nitrite concentrations. With this amount of ammonia added - NO2 concentrations around 20 - 30 ppm are not unlikely.
In the third article - they used 1mM witch respond to 46 mg/L and found NO2 concentrations around 0.2-0.4 mM = 9.2 - 18,4 mg/L in the blood stream. If I have done my calculation wrong- please let me know. If this is linear - it means that around 20 to 40 % of the ambient nitrite concentrations end up in the blood stream even among saltwater species. And the fact that the NO2 concentration in the blood is between 60 and 80 % lower than ambient concentration indicate an active transport out - probably through the chloride cells in the gills
Sincerely Lasse
- Joined
- Aug 24, 2016
- Messages
- 1,499
- Reaction score
- 2,293
Yes, Lasse but in freshwater fish it is much, much moreIf this is linear - it means that around 20 to 40 % of the ambient nitrite concentrations end up in the blood stream even among saltwater species.
This is two orders of magnitude difference.Kroupova et al.
Nitrite concentrations in the blood plasma may be more than 60 times higher than the concentrations in the surrounding medium (Fontenot and Isely, 1999).
Similar threads
- Replies
- 11
- Views
- 297
- Replies
- 10
- Views
- 297
- Replies
- 1
- Views
- 133
- Replies
- 5
- Views
- 343
- Replies
- 7
- Views
- 153
New Posts
-
-
-
DIY Ammonia dosing for low nitrate systems
- Latest: Randy Holmes-Farley
-
-


















