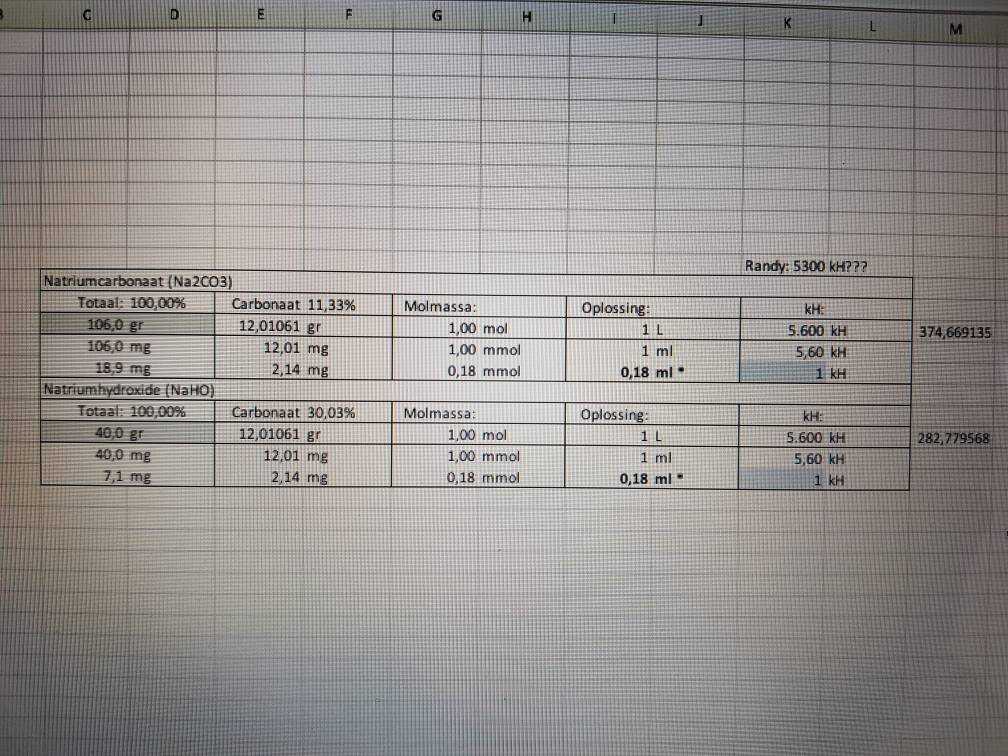
You can get bulk Sodium Hydroxide at Duda diesel.You can buy your micron filter socks there to.
https://www.dudadiesel.com/biodiesel_chemicals.php
That's who I bought the sodium sulfate from as well for the "other" high ph 2 part mix via Amazon. (Duda Energy)
Good link!