Thanks RandyThere are many kinds that should be unaffected by pH, especially polyethylene tubing, but i would have though even normal airline tubing was pretty resistant.
This sort of table can help pick tubing:
Tubing Chemical Compatibility Tables
http://www.hollandapt.com/Documents...ng_Chemcial_Compatability_uid172010827381.pdf
Drop down to the listing for
Sodium hydroxide, 25%
On page 2. Many types show as OK, but some are not.
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Second New DIY Two Part Recipe with Higher pH Boost
- Thread starter Randy Holmes-Farley
- Start date
- Tagged users None
- Joined
- Feb 21, 2017
- Messages
- 497
- Reaction score
- 248
Yes. CO2 will try to enter the alk part from the air, so keep it tightly closed.
Thanks again.
- Joined
- Apr 22, 2017
- Messages
- 512
- Reaction score
- 235
This might be a dumb question but can i use just the alk solution to help with ph and continue to use my current cal and mag (esv)
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,438
- Reaction score
- 63,831
This might be a dumb question but can i use just the alk solution to help with ph and continue to use my current cal and mag (esv)
In general, yes. What brand of calcium?
- Joined
- Apr 22, 2017
- Messages
- 512
- Reaction score
- 235
B ionicIn general, yes. What brand of calcium?
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,438
- Reaction score
- 63,831
That is an OK substitution until the ESV calcium runs out, but that calcium product is more potent than my DIY.
- Joined
- Apr 22, 2017
- Messages
- 512
- Reaction score
- 235
Ok so best to just use the esv calcium chloride recipe with the alk recipe.That is an OK substitution until the ESV calcium runs out, but that calcium product is more potent than my DIY.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,438
- Reaction score
- 63,831
Ok so best to just use the esv calcium chloride recipe with the alk recipe.
Not sure what you mean, but expect to dose less of the ESV calcium than the DIY recipe alk part. It would no longer be 1:1.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,438
- Reaction score
- 63,831
I discuss issues around determining how much strontium might be desirable to add to a recipe like this beginning here:
https://www.reef2reef.com/threads/n...h-higher-ph-boost.344500/page-10#post-4456604
https://www.reef2reef.com/threads/n...h-higher-ph-boost.344500/page-10#post-4456604
- Joined
- Aug 15, 2016
- Messages
- 285
- Reaction score
- 90
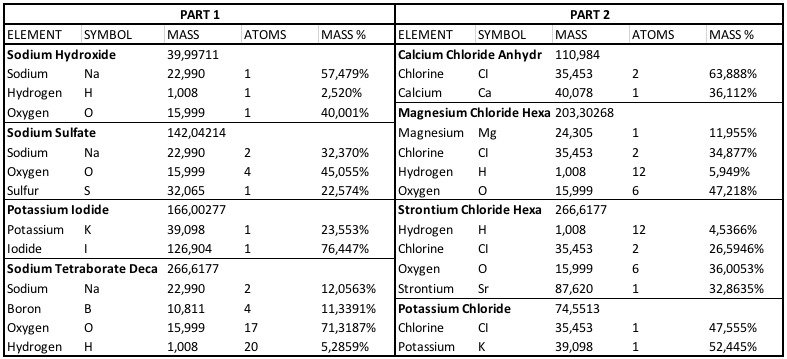
If you are aiming for 1:50 mole ratio; if you use calcium chloride dehydrate 55,5gr would be 20 gr calcium / 40 gr ( = 1 mole calcium) which is 0.5 mole. 50% of this is 0,02 x 0,5 mole = x mole strontium. 1 mole of strontium is 87,62 gram. x mole x 87,62 = x gram strontium. x gram strontium / mass % (32,863%) = 2,6 gram of strontium chloride hexahydrate... this does not include the amount lost which the amount of water removed to compensate for the rise of salinity. Maybe this overview may help you out;If i want to add strontium chloride in the cal/mag part, howmuch do i add? I know u dont support strontium, but i i want to add as iv been using seachem reef adv cal.
Last edited:
- Joined
- Apr 12, 2016
- Messages
- 1,873
- Reaction score
- 364
500 g of calcium chloride dihydrate plus 261.2 g of magnesium chloride hexahydrate and 8.3grams of strontium chloride in a total of 1 gallon..If you are aiming for 1:50 mole ratio; if you use calcium chloride dehydrate 55,5gr would be 20 gr calcium / 40 gr ( = 1 mole calcium) which is 0.5 mole. 50% of this is 0,02 x 0,5 mole = x mole strontium. 1 mole of strontium is 87,62 gram. x mole x 87,62 = x gram strontium. x gram strontium / mass % (32,863%) = 2,6 gram of strontium chloride hexahydrate... this does not include the amount lost which the amount of water removed to compensate for the rise of salinity.
THIS IS WHAT RANDY APPROVED IN THE OLDER 2part post.
- Joined
- Aug 15, 2016
- Messages
- 285
- Reaction score
- 90
55.5 anhydrate = 73,5 dihydrate for 2.6gr strontium. 500 / 73.5 = a factor of 6.8 x 2.6 = 17.7 gr. This is identical to Balling & DSR Easy ratio for strontium. The ratio of randy is not 1:50 but more towards 1:100.500 g of calcium chloride dihydrate plus 261.2 g of magnesium chloride hexahydrate and 8.3grams of strontium chloride in a total of 1 gallon..
THIS IS WHAT RANDY APPROVED IN THE OLDER 2part post.
- Joined
- Apr 12, 2016
- Messages
- 1,873
- Reaction score
- 364
Im confused, so do u add 2.6g or 17.7g? Instead of 8.3g55.5 anhydrate = 73,5 dihydrate for 2.6gr strontium. 500 / 73.5 = a factor of 6.8 x 2.6 = 17.7 gr. This is identical to Balling & DSR Easy ratio for strontium. The ratio of randy is not 1:50 but more towards 1:100.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,438
- Reaction score
- 63,831
My recommendation is here:
https://www.reef2reef.com/threads/n...h-higher-ph-boost.344500/page-10#post-4456604
8.3 grams of the strontium chloride hexahydrate per gallon. There's not going to be a perfect answer.
https://www.reef2reef.com/threads/n...h-higher-ph-boost.344500/page-10#post-4456604
8.3 grams of the strontium chloride hexahydrate per gallon. There's not going to be a perfect answer.
- Joined
- Aug 15, 2016
- Messages
- 285
- Reaction score
- 90
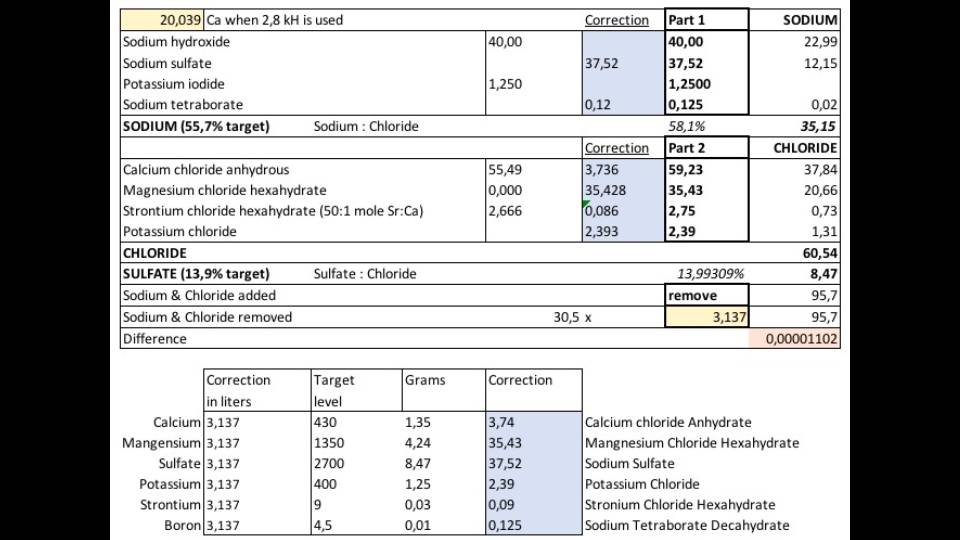
Wel.. I will add a bit more than 2.6gr per 55.5gr (for dihydrate 2.6 per 73,5gr) as I will also compensate for the salinity correction. I am not going to exactly follow the new recipe as I am missing a lot of sulfate in the recipe to compensate for the amounts removed with the salinity correction.. (randy is this looking at this I think). I will probably do this (also including trace elements)Im confused, so do u add 2.6g or 17.7g? Instead of 8.3g
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,438
- Reaction score
- 63,831
. I am not going to exactly follow the new recipe as I am missing a lot of sulfate in the recipe to compensate for the amounts removed with the salinity correction..
Can you clarify what that sentence means?
You add less sulfate to offset sulfate that is being lost in the salinity correction? That doesn't seem right, does it?
- Joined
- Aug 15, 2016
- Messages
- 285
- Reaction score
- 90
Can you clarify what that sentence means?
You add less sulfate to offset sulfate that is being lost in the salinity correction? That doesn't seem right, does it?
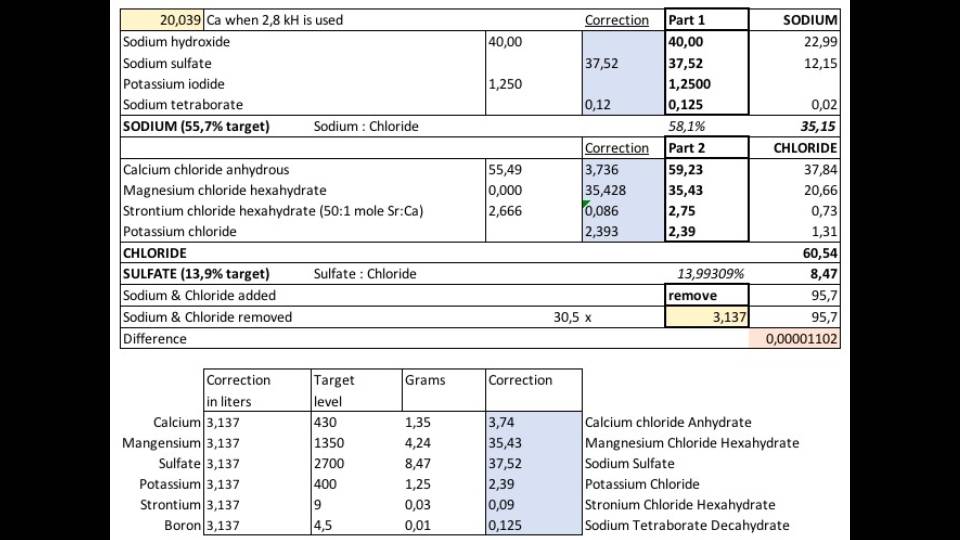
I will add much more sulfate. 37,52 gr per 40gr sodium hydroxide to be exact. This 37,52gr contains 8,47 gr of sulfate. which is 14% of the 60,54gr Chloride which is added by the stock solution. Around 3.1 L of aquariumwater is removed by the end of dosing this 1 liter of this stock (depends on the concentration but the principle remains the same). Apart from 60,54 chloride 35.15gr of sodium is added.. in total 95,7 gr of Sodium + chloride... 3.1l aquariumwater contains 30,5 x 3.1 = 95,7 gr of sodium & chloride.
There will be 3.1 l removed per 1l stock to keep salinity stable (again just to illustrate the calculation).. 3.1l contains;
3.1 x 2700 sulfate = 8,47 gr / 0,22574 = 37,52 gr of sodium sulfate. I believe this is accurate:
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,438
- Reaction score
- 63,831
This will get very confusing since the sulfate discussion is now appended to a recipe thread that does not include sodium sulfate. I'll continue that discussion on the other thread.
Similar threads
- Replies
- 1
- Views
- 76
- Replies
- 5
- Views
- 438
-
- AMS: Article
- Replies
- 61
- Views
- 4,189
- Replies
- 14
- Views
- 494
















