Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Whats your take on PH
- Thread starter Duffer
- Start date
- Tagged users None
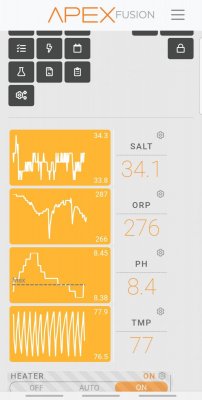
Speaking of PH I’m stuck at 7.8 in basement. I did the ph test with an air stone and it raised my PH so I am considering running a 1/2” pvc outside and step down to 1/4” then attach to my skimmer. I attached a picture of my skimmer. Do I attach the line to this muffler for the outside fresh air or should I remove the muffler and attach to the hose
Thanks

Thanks

Last edited:
Brew12
Electrical Gru
View BadgesExcellence Award
Reef Tank 365
Article Contributor
Moderator Emeritus
North Alabama Reef Club
Article Administrator
My Tank Thread
Yup, attach it to the top of the muffler.Speaking of PH I’m stuck at 7.8 in basement. I did the ph test with an air stone and it raised my PH so I am considering running a 1/2” pvc outside and step down to 1/4” then attach to my skimmer. I attached a picture of my skimmer. Do I attach the line to this muffler for the outside fresh air or should I remove the muffler and attach to the hose
Thanks

Thank you much!Yup, attach it to the top of the muffler.
Taking outside air is a fantastic option if you can, but if you can not then we have to look for another alternative especially if run a calc reactor which further suppresses PH.
Two options available are kalkwasser or scrubbers.
Two options available are kalkwasser or scrubbers.
I did the Kalwasser on my smaller tank and had a mishap that ended up dumping 5 gallons of kalk water in the tank turning it into milk. Fortunately I only lost a feather duster but that scared me. Now I am going to look at other alternatives. The fresh air shouldn’t be too bad the run is only about 10ft for my tank. I’m sure I can pull enough air to get my ph to about 8.1. And I see scrubbers can get expensive with the media
I have an airpump outside that pumps several lines into my sump. I find it easier than using the skimmer, but my sump is a 180 gallon tank. 
Never test for it so I can’t offer anything 
Dear god. I’m only feeding a 20 gallon sump for a 40 gallon tank. Guess a good air pump would be another alternative than the skimmer. What air pump are you using?I have an airpump outside that pumps several lines into my sump. I find it easier than using the skimmer, but my sump is a 180 gallon tank.
I have a question or two addressing the indoor air only option for CO2 removal prior to the skimmer concerning PH control.
1. If the CO2 scrubber is a viable option. What is the most economical media you have found?
2. Assuming the scrubber media removes more than CO2, (i.e. air borne pollutants), would the addition of activated coconut carbon help with that? I am assuming it would not affect the efficiency of the CO2 media.
3.Would just spraying Febreze over the tank remove all air borne concerns? Just kidding.
1. If the CO2 scrubber is a viable option. What is the most economical media you have found?
2. Assuming the scrubber media removes more than CO2, (i.e. air borne pollutants), would the addition of activated coconut carbon help with that? I am assuming it would not affect the efficiency of the CO2 media.
3.Would just spraying Febreze over the tank remove all air borne concerns? Just kidding.
vetteguy53081
Well known Member and monster tank lover
View Badges
Partner Member 2024
Excellence Award
Reef Tank 365
RGB
Article Contributor
Tampa Bay Reef Keepers
West Palm Beach Reefer
Hospitality Award
Ocala Reef Club Member
305 Reef Club
Wisco Reefers
Midwest Reefer
Fish Medic
MAC of SW Florida
Rock Pool Reef Keepers
R2R Secret Santa 2023
My Tank Thread
My Aquarium Showcase
I know where I want mine to be but I am more interested in a stable PH than a target and will NOT chase my numbers or dosages to make it be perfect as it will never be.
I just installed a calcium reactor and interested to see what effect it has in a more stable reading
I just installed a calcium reactor and interested to see what effect it has in a more stable reading
My ph 8.0-8.1, but my tank is softies and is outside. Plus no sunlight hits tank.That´s what we think at least but controlled experiment that i have been a part of (unpublished for the moment) indicate something else. There seems to be a "sweet point" around 8.1 - 8.15 there the calcification rate and CO2 concentrations in the water seems to be optimal for growth of at least hystrix and montipora sp. This is also in line with experiences with aquarium with calcium reactors and growth - they often operate between 7.9 and 8.2
I both agree and disagree. If you use some type of additives and use Na2CO3 instead of NaHCO3 as alkalinity part - you can easily hinder a too low dip during night time. This graph shows my aquarium the last days. The sawtooth graph between 20:00 and 12:00 is caused by my just adding my Core 7 3a + b between these times. It also show - IMO - the most important reason to general pH drip (or low maximal top) Around 16;00 yesterday . two of my grandchildren show up - and they are still here
Sincerely Lasse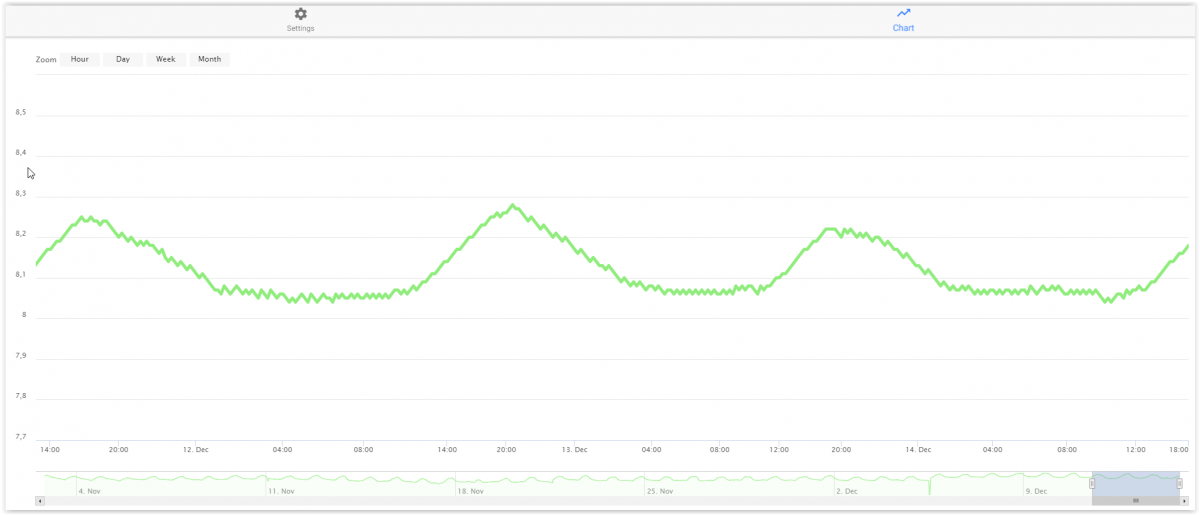
Where I live we get smoke in the air several times a year due to the burning of sugar cane fields and I swear I smell the Burger King down the road at night in my house. CO2 may not be an issue in my old house, I'm pretty sure I get 100% outside air exchange every 24 hours.Would the Ph stabilize utilizing outside air? Are there any concerns which would cause a fluctuation As far as weather?
Would the Ph stabilize utilizing outside air? Are there any concerns which would cause a fluctuation As far as weather?
I also run my skimmer outside (I've connected it directly to the venturi), and run a fuge 10% the size of my tank (54g) with a 150W grow light 20hrs a day. I also use kalk in my ato. My pH this way swings between 7.7 - 8.1 night vs. day.
I do have a CaRx on constant flow, efluent at pH 6.4, but I run it only when the main lights are on.
What I want to say is, that sometimes even though all the wisdom, it just doesn't work out.. I'm pretty much considering going back to balling now after a year, as I've had better results with that, and I think it was mainly because of the pH.
If you do not live in on of the mega city´s of the world - outside air can be a alternative - but in cities - maybe not a good idea - either no good idea if your home is located near commercial growing fields of the "dirty dozen".
But I think that I just read a game changer about which parameter is most important according to calcification in at least some stony corals
@ksed tip me about a study publicized in 2014 that - IMO - can be a game changer how we treat our tanks in order to get a high calcification rate. It can also give some explanations why over sized skimmers and aquarium with a very low organic load can give high calcification rates.
It can also explain why my tank seems to go very good even if I dose all my alkalinity during night.
It can also explain some good experiences with oxydators and/or light reversed refugium.
It can also explain why some things we do in order to get a high pH (read low CO2)
It also strengthen my believe that the method using recirculated skimmer air (through a carbon scrubber) is a real cerebral hemorrhage - it will give a high pH but no exchange of other gases like O2 canf NH3 .
In their experiment - they did not see any differences between light and dark calcification if some parameters was right.
To really understand this - we must understand that O2 and CO2 is two different sides of the same coin according to biological life and aeration in a reef aquarium. And it is only the amount of CO2 that affect the pH - not the amount of O2.
During night time - nearly all life in the aquarium use 02 and produce CO2. - this will give a lower O2 and a higher CO2 pressure in the water. The O2 level will be below equilibrium of air/water and the CO2 will be higher than the equilibrium. Heavy aeration (by a skimmer, gas exchanges tower or other types of aeration of water) will rise the O2 level and lower the CO2 level (hence rise the pH). During the photo period all animals still consume O2 and leave CO2 as a waste but all primary producers (algae, some sponges and the zooxanthellae in some animals) will use CO2 and produce O2 with help of light. when consumption of CO2 is higher than the production of CO2 - pH rise and so do O2. Heavy aeration by a skimmer, gas exchanges tower or other types of aeration of water will rise the CO2 (hence stabilize the pH) and decrease the O2.
With this in my head this quote from the article rise new ideas and thinking
The article
Sincerely Lasse
But I think that I just read a game changer about which parameter is most important according to calcification in at least some stony corals
@ksed tip me about a study publicized in 2014 that - IMO - can be a game changer how we treat our tanks in order to get a high calcification rate. It can also give some explanations why over sized skimmers and aquarium with a very low organic load can give high calcification rates.
It can also explain why my tank seems to go very good even if I dose all my alkalinity during night.
It can also explain some good experiences with oxydators and/or light reversed refugium.
It can also explain why some things we do in order to get a high pH (read low CO2)
It also strengthen my believe that the method using recirculated skimmer air (through a carbon scrubber) is a real cerebral hemorrhage - it will give a high pH but no exchange of other gases like O2 canf NH3 .
In their experiment - they did not see any differences between light and dark calcification if some parameters was right.
To really understand this - we must understand that O2 and CO2 is two different sides of the same coin according to biological life and aeration in a reef aquarium. And it is only the amount of CO2 that affect the pH - not the amount of O2.
During night time - nearly all life in the aquarium use 02 and produce CO2. - this will give a lower O2 and a higher CO2 pressure in the water. The O2 level will be below equilibrium of air/water and the CO2 will be higher than the equilibrium. Heavy aeration (by a skimmer, gas exchanges tower or other types of aeration of water) will rise the O2 level and lower the CO2 level (hence rise the pH). During the photo period all animals still consume O2 and leave CO2 as a waste but all primary producers (algae, some sponges and the zooxanthellae in some animals) will use CO2 and produce O2 with help of light. when consumption of CO2 is higher than the production of CO2 - pH rise and so do O2. Heavy aeration by a skimmer, gas exchanges tower or other types of aeration of water will rise the CO2 (hence stabilize the pH) and decrease the O2.
With this in my head this quote from the article rise new ideas and thinking
Interestingly, we did not find evidence for the theory of light-enhanced calcification (Kawaguti and Sakumoto, 1948; Chalker and Taylor, 1975) at baseline conditions of pH 8.1 and normoxia, as light calcification rates were lower than dark calcification rates. This observation is in agreement with a recent study on the growth of Galaxea fascicularis, during which no significant difference between light and dark calcification rates was found when the incubation water was aerated to maintain normoxia (Wijgerde et al., 2012), although it contrasts with several previous studies (reviewed by Gattuso et al., 1999). It seems that light-enhanced calcification is only found when dark calcification rates are impaired due to oxygen limitation and/or reduced oxygen mass transfer at low water flow. It must be noted, however, that the light and dark experiments were carried out with an interlude of several weeks. It is therefore possible that other time-dependent factors in the holding aquarium were responsible for the observed differences between the light and dark experiments.
The article
Sincerely Lasse
^This is a great and interesting thought, BUT  )):
)):
In a normal home aquarium it is in my opinion not possible to get an oxygen low enviroment, at least not in the water column.
See, the gas-water exchange surface is so large & with the powerheads creating flow and constantly stirring our tanks, the O2 levels can't really drop a lot even during the night (when calcification halts or at least is reduced greatly anyway because of the lack of the light). You'd need at least a 5-6ft deep aquarium & now flow to start losing oxygen on the bottom.
I've been doing this most of my life, although in fresh-water, on lakes, and the only way to lose significant oxygen (even there) is if there are (AND condition)
a, no surface movement (wind)
b, there is already an algae issue, like an algae bloom in the water column -> that way the nights are rough as the algae consumes all of the O2 ~3-4ft and more below the surface
c, high temperatures (80f +), which I believe limits the amount of oxygen can dissolve
Interestingly enough, if one introduces water movement into such a lake, and interestingly there isn't much movement needed, the problem goes away very fast. So that's why I think that it is not really possible to have O2 problems in a reef tank.
In a normal home aquarium it is in my opinion not possible to get an oxygen low enviroment, at least not in the water column.
See, the gas-water exchange surface is so large & with the powerheads creating flow and constantly stirring our tanks, the O2 levels can't really drop a lot even during the night (when calcification halts or at least is reduced greatly anyway because of the lack of the light). You'd need at least a 5-6ft deep aquarium & now flow to start losing oxygen on the bottom.
I've been doing this most of my life, although in fresh-water, on lakes, and the only way to lose significant oxygen (even there) is if there are (AND condition)
a, no surface movement (wind)
b, there is already an algae issue, like an algae bloom in the water column -> that way the nights are rough as the algae consumes all of the O2 ~3-4ft and more below the surface
c, high temperatures (80f +), which I believe limits the amount of oxygen can dissolve
Interestingly enough, if one introduces water movement into such a lake, and interestingly there isn't much movement needed, the problem goes away very fast. So that's why I think that it is not really possible to have O2 problems in a reef tank.
Last edited:
IMO - it is more common than we believe. It depends of the biomass in the system. In planted aquarium (freshwater) it is a accepted fact that dense planted aquariums can have oxygen levels as low as it will kill fish. In a dens populated aquarium with mushrooms and Xenia - without skimmer - I had a major fish kill during early morning - probably because of oxygen depletion.In a normal home aquarium it is in my opinion not possible to get an oxygen low enviroment, at least not in the water column.
General pattern of Oxygen levels you can find in this article
But the authors do not talk about total oxygen deficiency - one of the experiments was run at 30% O2 saturation in 26 degree C and saltwater - it correspond to around 1.9 mg O2/l.
IMO - low oxygen concentration is more common in saltwater compared with freshwater because 100 % saturation is not the same for the types of water. !00 % saturation in freshwater (26 C) correspond to 8.12 mg/l O2 and 100 % saturation in saltwater (26 C - 35 psu) correspond to around 6.36 mg/l O2. - A 80 % saturation in saltwater (26 C - 35 psu) correspond to around 5 mg/l O2 - rather near the oxygen level that are know to diminish the nitrite oxidation rate.
This is interesting because this article shows that there is not any significant difference between dark and light calcification if the oxygen saturation is 100 % (at least for the species studied in this article) And they show also that calcification rate for the pH 8.4 is very depended on the oxygen saturation - at 170 % saturation it is the same as at pH 8.1. We shall also remember that it is often created micro environment around our corals there the oxygen saturation can be very high. A very high flow will help to decrease the risk for such micro environment.(when calcification halts or at least is reduced greatly anyway because of the lack of the light)
This diagram from the article show very interesting results
Probably I can speculate for 4 pages what this means but it is still only one investigation (referring to a couple of others). It is only valid for this specimens and in this study but it surly indicate rather interesting ideas and that can explain a lot of observations I have done during the years.
The article indicate that it is more important to monitor oxygen levels in a reef aquarium that we have assumed before. The problem is that the normal oxygen probes from the companies delivering such probes is expensive and not so good or easy to use in a biological loaded system. However there is techniques today that make oxygen easier to monitor and I hope someone will pick up that techniques now when MindStream seems to close their doors.
Sincerely Lasse
Last edited:
According to lakes - it is not so easy to aerate them during summer - and in the rest of the year especially not the lakes that´s not in the temperate zone. The term thermocline and "spring and autumn stirring" can explain that. For the moment - the deepest freshwater lake in the world Lake Baikal - have oxygen all way down to 1642 meters but Lake Tanganyika (the second deepest) only have oxygen down to around 200 m (1470 m deep). Lake Victoria was oxygenated all the way to the bottom (83 m) but because of eutrophication - the lake has developed deep water with oxygen deficiency since the middle of the 1980:ties.
Sincerely Lasse
Sincerely Lasse
easiest way to keep PH stable if you have a sump is to cut your return pipe enough above your sock, or termination into your sump that it has an air gap. This forces the return water to be aerated, which will prevent PH drops , a skimmer will also do this if you have outside air fed to your venturi. Mine stays at stable 8.4 with 9 dkh his way
Similar threads
- Replies
- 6
- Views
- 148